Venezuelan equine encephalitis virus capsid protein inhibits nuclear import in Mammalian but not in mosquito cells
- PMID: 18256144
- PMCID: PMC2293000
- DOI: 10.1128/JVI.02330-07
Venezuelan equine encephalitis virus capsid protein inhibits nuclear import in Mammalian but not in mosquito cells
Abstract
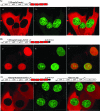
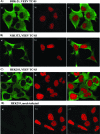
Venezuelan equine encephalitis virus (VEEV) represents a continuous public health threat in the United States. It has the ability to cause fatal disease in humans and in horses and other domestic animals. We recently demonstrated that replicating VEEV interferes with cellular transcription and uses this phenomenon as a means of downregulating a cellular antiviral response. VEEV capsid protein was found to play a critical role in this process, and its approximately 35-amino-acid-long peptide, fused with green fluorescent protein, functioned as efficiently as did the entire capsid. We detected a significant fraction of VEEV capsid associated with nuclear envelope, which suggested that this protein might regulate nucleocytoplasmic trafficking. In this study, we demonstrate that VEEV capsid and its N-terminal sequence efficiently inhibit multiple receptor-mediated nuclear import pathways but have no effect on the passive diffusion of small proteins. The capsid protein of the Old World alphavirus Sindbis virus and the VEEV capsid, with a previously defined frameshift mutation, were found to have no detectable effect on nuclear import. Importantly, the VEEV capsid did not noticeably interfere with nuclear import in mosquito cells, and this might play a critical role in the ability of the virus to develop a persistent, life-long infection in mosquito vectors. These findings demonstrate a new aspect of VEEV-host cell interactions, and the results of this study are likely applicable to other New World alphaviruses, such as eastern and western equine encephalitis viruses.
Figures
References
-
- Alevizatos, A. C., R. W. McKinney, and R. D. Feigin. 1967. Live, attenuated Venezuelan equine encephalomyelitis virus vaccine. I. Clinical effects in man. Am. J. Trop. Med. Hyg. 16762-768. - PubMed
-
- Berge, T. O., I. S. Banks, and W. D. Tigertt. 1961. Attenuation of Venezuelan equine encephalomyelitis virus by in vitro cultivation in guinea pig heart cells. Am. J. Hyg. 73209-218.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

