The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes
- PMID: 18256157
- PMCID: PMC2292992
- DOI: 10.1128/JVI.01818-07
The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes
Abstract
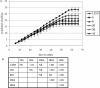
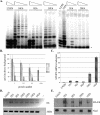
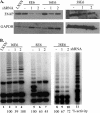
Human papillomaviruses (HPVs) belonging to the Betapapillomavirus genus have recently been implicated in squamous cell carcinomas of the skin, though the mechanisms by which they initiate carcinogenesis are unclear. We show that human foreskin keratinocytes (HFKs) expressing several betapapillomavirus E6 (beta-E6) proteins display life span extension, but not to the extent seen in HFKs expressing HPV type 16 E6 (16E6). Additionally, we demonstrate that beta-E6 proteins can differentially activate telomerase. HFKs expressing 38E6 exhibit significant telomerase activity but to a lesser degree than that observed with 16E6; however, other beta-E6 proteins, including 5E6, 8E6, 20E6, and 22E6, exhibit low or background levels of telomerase activity. Utilizing glutathione S-transferase pull-down and coimmunoprecipitation experiments, the beta-E6 proteins were shown to interact with the cellular proteins E6-associated protein (E6AP) and NFX1-91, two proteins known to be important for telomerase activation by 16E6. Interestingly, the relative strength of the interaction between E6 and E6AP or NFX1-91 was proportionate to the activation of telomerase by each beta-E6 protein. To address the requirement for E6AP in telomerase activation by beta-E6 proteins, we utilized a shRNA to knock down endogenous levels of E6AP. Lysates with decreased levels of E6AP showed a reduced ability to activate telomerase, suggesting that E6AP is a necessary component. These data suggest that complex formation between E6, E6AP, and NFX1-91 is a critical step in mediating telomerase activation, which may be one contributing factor to cellular life span extension during human betapapillomavirus infection.
Figures

References
-
- Bartz, S. R., and M. A. Vodicka. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12337-342. - PubMed
-
- Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 564620-4624. - PubMed
-
- Caldeira, S., R. Filotico, R. Accardi, I. Zehbe, S. Franceschi, and M. Tommasino. 2004. p53 mutations are common in human papillomavirus type 38-positive non-melanoma skin cancers. Cancer Lett. 209119-124. - PubMed
-
- Chung, H. K., C. Cheong, J. Song, and H. W. Lee. 2005. Extratelomeric functions of telomerase. Curr. Mol. Med. 5233-241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

