Self-assembly of the plant cell wall requires an extensin scaffold
- PMID: 18256186
- PMCID: PMC2538902
- DOI: 10.1073/pnas.0711980105
Self-assembly of the plant cell wall requires an extensin scaffold
Abstract
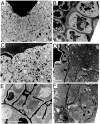
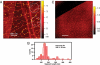
Cytokinesis partitions the cell by a cleavage furrow in animals but by a new cross wall in plants. How this new wall assembles at the molecular level and connects with the mother cell wall remains unclear. A lethal Arabidopsis embryogenesis mutant designated root-, shoot-, hypocotyl-defective (rsh) provides some clues: RSH encodes extensin AtEXT3, a structural glycoprotein located in the nascent cross wall or "cell plate" and also in mature cell walls. Here we report that electron micrographs of rsh mutant cells lacking RSH extensin correspond to a wall phenotype typified by incomplete cross wall assembly. Biochemical characterization of the purified RSH glycoprotein isolated from wild-type Arabidopsis cell cultures confirmed its identity as AtEXT3: a (hydroxy)proline-rich glyco protein comprising 11 identical amphiphilic peptide repeats with a 28-residue periodicity: SOOOOKKHYVYKSOOOOVKHYSOOOVYH (O = Hyp), each repeat containing a hydrophobic isodityrosine cross-link motif (YVY, underlined). Atomic force microscopy of RSH glycoprotein imaged its propensity for self-assembly into a dendritic scaffold. Extensin peroxidase catalyzed in vitro formation of insoluble RSH gels with concomitant tyrosine cross-linking, hence this likelihood in muro. We conclude that self-assembling amphiphiles of lysine-rich RSH extensin form positively charged scaffolds in the cell plate. These react with negatively charged pectin to create an extensin pectate coacervate that may template further orderly deposition of the new cross wall at cytokinesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lamport DTA. The protein component of primary cell walls. Adv Bot Res. 1965;2:151–218.
-
- Epstein L, Lamport DTA. An intramolecular linkage involving isodityrosine in extensin. Phytochemistry. 1984;23:1241–1246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

