Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells
- PMID: 18256257
- PMCID: PMC6671574
- DOI: 10.1523/JNEUROSCI.3632-07.2008
Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells
Abstract
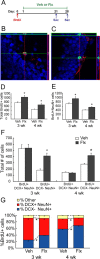
Chronic treatments with selective serotonin reuptake inhibitors (SSRIs) have been shown to increase hippocampal neurogenesis. However, it is not known whether SSRIs impact the maturation and functional integration of newborn neurons. Here we examined the effects of subchronic and chronic fluoxetine on the structural and physiological properties of young granule cells. Our results show that doublecortin-positive immature neurons displayed increased dendritic arborization after chronic fluoxetine treatment. In addition, chronic but not subchronic fluoxetine elicited a decrease in the number of newborn neurons expressing immature markers and a corresponding increase in those expressing mature markers. These results suggest that chronic fluoxetine accelerates the maturation of immature neurons. We also investigated the effects of fluoxetine on a form of neurogenesis-dependent long-term potentiation (LTP) in the dentate gyrus. This form of LTP was enhanced by chronic fluoxetine, and ablation of neurogenesis with x-irradiation completely blocked the effects of chronic fluoxetine on LTP. Finally, we demonstrated that the behavioral effect of fluoxetine in the novelty-suppressed feeding test requires chronic administration and is blocked by x-irradiation. These results show that the effects of fluoxetine on LTP and behavior both require neurogenesis and follow a similar delayed time course. The effects of chronic fluoxetine on the maturation and functional properties of young neurons may therefore be necessary for its anxiolytic/antidepressant activity and contribute to its delayed onset of therapeutic efficacy.
Figures






References
-
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. - PubMed
-
- American Psychiatric Association. The practice of ECT: recommendations for treatment, training and privileging. Convuls Ther. 1990;6:85–120. - PubMed
-
- Anderson GM, Barr CS, Lindell S, Durham AC, Shifrovich I, Higley JD. Time course of the effects of the serotonin-selective reuptake inhibitor sertraline on central and peripheral serotonin neurochemistry in the rhesus monkey. Psychopharmacology (Berl) 2005;178:339–346. - PubMed
-
- Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology (Berl) 1989;97:277–279. - PubMed
-
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
