A synaptic basis for auditory-vocal integration in the songbird
- PMID: 18256272
- PMCID: PMC6671566
- DOI: 10.1523/JNEUROSCI.3838-07.2008
A synaptic basis for auditory-vocal integration in the songbird
Abstract
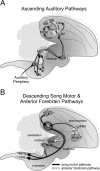
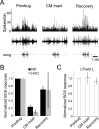
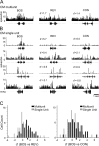
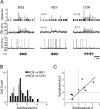
Songbirds learn to sing by memorizing a tutor song that they then vocally mimic using auditory feedback. This developmental sequence suggests that brain areas that encode auditory memories communicate with brain areas for learned vocal control. In the songbird, the secondary auditory telencephalic region caudal mesopallium (CM) contains neurons that encode aspects of auditory experience. We investigated whether CM is an important source of auditory input to two sensorimotor structures implicated in singing, the telencephalic song nucleus interface (NIf) and HVC. We used reversible inactivation methods to show that activity in CM is necessary for much of the auditory-evoked activity that can be detected in NIf and HVC of anesthetized adult male zebra finches. Furthermore, extracellular and intracellular recordings along with spike-triggered averaging methods indicate that auditory selectivity for the bird's own song is enhanced between CM and NIf. We used lentiviral-mediated tracing methods to confirm that CM neurons directly innervate NIf. To our surprise, these tracing studies also revealed a direct projection from CM to HVC. We combined irreversible lesions of NIf with reversible inactivation of CM to establish that CM supplies a direct source of auditory drive to HVC. Finally, using chronic recording methods, we found that CM neurons are active in response to song playback and during singing, indicating their potential importance to song perception and processing of auditory feedback. These results establish the functional synaptic linkage between sites of auditory and vocal learning and may identify an important substrate for learned vocal communication.
Figures
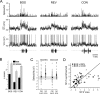

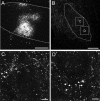
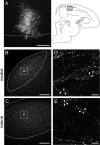
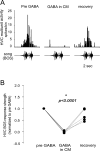

References
-
- Amin N, Grace JA, Theunissen FE. Neural response to bird's own song and tutor song in the zebra finch field L and caudal mesopallium. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:469–489. - PubMed
-
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. - PubMed
-
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. - PubMed
-
- Cardin JA, Schmidt MF. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. J Neurophysiol. 2003;90:2884–2899. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
