Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors
- PMID: 18256283
- PMCID: PMC2291422
- DOI: 10.1091/mbc.e07-09-0942
Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors
Abstract
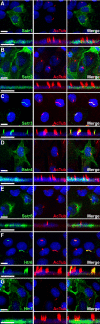
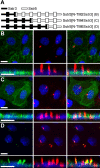
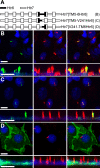
Primary cilia are sensory organelles present on most mammalian cells. The functions of cilia are defined by the signaling proteins localized to the ciliary membrane. Certain G protein-coupled receptors (GPCRs), including somatostatin receptor 3 (Sstr3) and serotonin receptor 6 (Htr6), localize to cilia. As Sstr3 and Htr6 are the only somatostatin and serotonin receptor subtypes that localize to cilia, we hypothesized they contain ciliary localization sequences. To test this hypothesis we expressed chimeric receptors containing fragments of Sstr3 and Htr6 in the nonciliary receptors Sstr5 and Htr7, respectively, in ciliated cells. We found the third intracellular loop of Sstr3 or Htr6 is sufficient for ciliary localization. Comparison of these loops revealed a loose consensus sequence. To determine whether this consensus sequence predicts ciliary localization of other GPCRs, we compared it with the third intracellular loop of all human GPCRs. We identified the consensus sequence in melanin-concentrating hormone receptor 1 (Mchr1) and confirmed Mchr1 localizes to primary cilia in vitro and in vivo. Thus, we have identified a putative GPCR ciliary localization sequence and used this sequence to identify a novel ciliary GPCR. As Mchr1 mediates feeding behavior and metabolism, our results implicate ciliary signaling in the regulation of body weight.
Figures



References
-
- Badano J. L., Mitsuma N., Beales P. L., Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 2006;7:125–148. - PubMed
-
- Berbari N. F., Bishop G. A., Askwith C. C., Lewis J. S., Mykytyn K. Hippocampal neurons possess primary cilia in culture. J. Neurosci. Res. 2007;85:1095–1100. - PubMed
-
- Bisgrove B. W., Yost H. J. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. - PubMed
-
- Bishop G. A., Berbari N. F., Lewis J. S., Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J. Comp. Neurol. 2007;505:562–571. - PubMed
-
- Bloodgood R. A. Protein targeting to flagella of trypanosomatid protozoa. Cell Biol. Int. 2000;24:857–862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

