A novel role for PA28gamma-proteasome in nuclear speckle organization and SR protein trafficking
- PMID: 18256291
- PMCID: PMC2291414
- DOI: 10.1091/mbc.e07-07-0637
A novel role for PA28gamma-proteasome in nuclear speckle organization and SR protein trafficking
Abstract
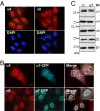
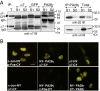
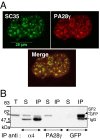
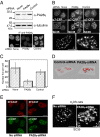
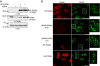
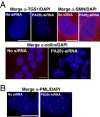
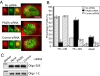
In eukaryotic cells, proteasomes play an essential role in intracellular proteolysis and are involved in the control of most biological processes through regulated degradation of key proteins. Analysis of 20S proteasome localization in human cell lines, using ectopic expression of its CFP-tagged alpha7 subunit, revealed the presence in nuclear foci of a specific and proteolytically active complex made by association of the 20S proteasome with its PA28gamma regulator. Identification of these foci as the nuclear speckles (NS), which are dynamic subnuclear structures enriched in splicing factors (including the SR protein family), prompted us to analyze the role(s) of proteasome-PA28gamma complexes in the NS. Here, we show that knockdown of these complexes by small interfering RNAs directed against PA28gamma strongly impacts the organization of the NS. Further analysis of PA28gamma-depleted cells demonstrated an alteration of intranuclear trafficking of SR proteins. Thus, our data identify proteasome-PA28gamma complexes as a novel regulator of NS organization and function, acting most likely through selective proteolysis. These results constitute the first demonstration of a role of a specific proteasome complex in a defined subnuclear compartment and suggest that proteolysis plays important functions in the precise control of splicing factors trafficking within the nucleus.
Figures




References
-
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. - PubMed
-
- Barton L. F., Runnels H. A., Schell T. D., Cho Y., Gibbons R., Tevethia S. S., Deepe G. S., Jr, Monaco J. J. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J. Immunol. 2004;172:3948–3954. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

