Immortalization of neural precursors when telomerase is overexpressed in embryonal carcinomas and stem cells
- PMID: 18256293
- PMCID: PMC2291397
- DOI: 10.1091/mbc.e06-11-1013
Immortalization of neural precursors when telomerase is overexpressed in embryonal carcinomas and stem cells
Abstract
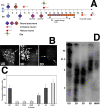
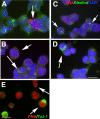
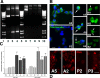
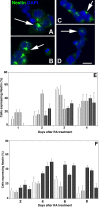
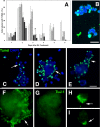
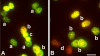
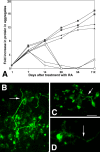
The DNA repair enzyme telomerase maintains chromosome stability by ensuring that telomeres regenerate each time the cell divides, protecting chromosome ends. During onset of neuroectodermal differentiation in P19 embryonal carcinoma (EC) cells three independent techniques (Southern blotting, Q-FISH, and Q-PCR) revealed a catastrophic reduction in telomere length in nestin-expressing neuronal precursors even though telomerase activity remained high. Overexpressing telomerase protein (mTERT) prevented telomere collapse and the neuroepithelial precursors produced continued to divide, but deaggregated and died. Addition of FGF-2 prevented deaggregation, protected the precursors from the apoptotic event that normally accompanies onset of terminal neuronal differentiation, allowed them to evade senescence, and enabled completion of morphological differentiation. Similarly, primary embryonic stem (ES) cells overexpressing mTERT also initiated neuroectodermal differentiation efficiently, acquiring markers of neuronal precursors and mature neurons. ES precursors are normally cultured with FGF-2, and overexpression of mTERT alone was sufficient to allow them to evade senescence. However, when FGF-2 was removed in order for differentiation to be completed most neural precursors underwent apoptosis indicating that in ES cells mTERT is not sufficient allow terminal differentiation of ES neural precursors in vitro. The results demonstrate that telomerase can potentiate the transition between pluripotent stem cell and committed neuron in both EC and ES cells.
Figures
References
-
- Alexiades M. R., Cepko C. Quantitative analysis of proliferation and cell cycle length during development of the rat retina. Dev. Dyn. 1996;205:293–307. - PubMed
-
- Andrews P. W., Matin M. M., Bahrami A. R., Damjanov I., Gokhale P., Draper J. S. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem. Soc. Trans. 2005;33:1526–1530. - PubMed
-
- Armstrong L., Lako M., Lincoln J., Cairns P. M., Hole N. mTERT expression correlates with telomerase activity during the differentiation of murine embryonic stem cells. Mech. Dev. 2000;97:109–116. - PubMed
-
- Armstrong L., Saretzki G., Peters H., Wappler I., Evans J., Hole N., von Zglinicki. T., Lako M. Overexpression of telomerase confers growth advantage, stress resistance and enhanced differentiation of ESCs toward the hematopoetic lineage. Stem Cells. 2005;23:516–529. - PubMed
-
- Astigiano S., Damonte P., Fossati S., Boni L., Barbieri O. Fate of embryonal carcinoma cells injected into postimplantation mouse embryos. Differentiation. 2005;73:484–490. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

