TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity
- PMID: 18256356
- PMCID: PMC2386719
- DOI: 10.1681/ASN.2007090982
TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity
Abstract
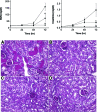
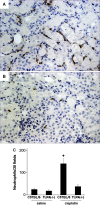
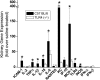
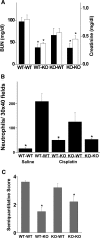
The molecular mechanisms of acute kidney injury (AKI) remain unclear. Toll-like receptors (TLRs), widely expressed on leukocytes and kidney epithelial cells, regulate innate and adaptive immune responses. The present study examined the role of TLR signaling in cisplatin-induced AKI. Cisplatin-treated wild-type mice had significantly more renal dysfunction, histologic damage, and leukocytes infiltrating the kidney than similarly treated mice with a targeted deletion of TLR4 [Tlr4(-/-)]. Levels of cytokines in serum, kidney, and urine were increased significantly in cisplatin-treated wild-type mice compared with saline-treated wild-type mice and cisplatin-treated Tlr4(-/-) mice. Activation of JNK and p38, which was associated with cisplatin-induced renal injury in wild-type mice, was significantly blunted in Tlr4(-/-) mice. Using bone marrow chimeric mice, it was determined that renal parenchymal TLR4, rather than myeloid TLR4, mediated the nephrotoxic effects of cisplatin. Therefore, activation of TLR4 on renal parenchymal cells may activate p38 MAPK pathways, leading to increased production of inflammatory cytokines, such as TNF-alpha and subsequent kidney injury. Targeting the TLR4 signaling pathways may be a feasible therapeutic strategy to prevent cisplatin-induced AKI in humans.
Figures




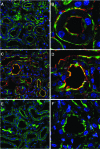
References
-
- Ries F, Klastersky J: Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis 13: 368–379, 1986 - PubMed
-
- Ramesh G, Reeves WB: TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 54: F610-F618, 2003 - PubMed
-
- Ramesh G, Reeves WB: p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol 289: F166-F174, 2005 - PubMed
-
- Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Matsutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara K, Hirakata H, Iida M: Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int 63: 72–82, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

