Preclinical studies in rats and squirrel monkeys for safety evaluation of the bivalent anti-human T cell immunotoxin, A-dmDT390-bisFv(UCHT1)
- PMID: 18256829
- PMCID: PMC11030202
- DOI: 10.1007/s00262-008-0457-x
Preclinical studies in rats and squirrel monkeys for safety evaluation of the bivalent anti-human T cell immunotoxin, A-dmDT390-bisFv(UCHT1)
Abstract
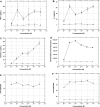
The bivalent anti-human T cell immunotoxin A-dmDT390-bisFv(UCHT1) for treatment of patients with T cell malignancies is a single chain fusion protein composed of the catalytic domain and translocation domains of diphtheria toxin fused to two tandem sFv molecules reactive with human CD3 epsilon. This immunotoxin selectively kills CD3 epsilon positive T cells. To determine the maximum tolerated dose (MTD), pharmacokinetics and immunogenicity of A-dmDT390-bisFv(UCHT1), rat and squirrel monkey studies were performed. In both animal studies, animals received either 0, 2.5 (low), 25 (medium), or 56.25 microg/kg (high) of A-dmDT390-bisFv(UCHT1) intravenously twice daily for four consecutive days. Although transient elevation of liver transaminases in the high groups was observed, the A-dmDT390-bisFv(UCHT1) administration did not affect liver function, renal function, the hemogram, or produce serious organ histopathology. Adverse events included transient lethargy, inappetence and weight loss in high groups. A-dmDT390-bisFv(UCHT1) plasma half life was 26.95 min in rats and 18.33 min in squirrel monkeys. Immune responses to A-dmDT390-bisFv(UCHT1) were minimal in squirrel monkeys and mild in rats. In vitro cytokine release, T cell activation and CD3 epsilon receptor occupancy assays using human PBMC were further performed since rat and squirrel monkey T cells do not react with A-dmDT390-bisFv(UCHT1). A-dmDT390-bisFv(UCHT1) did not induce cytokine release or T cell activation. The A-dmDT390-bisFv(UCHT1) concentration for 50% CD3 epsilon receptor occupancy was 7.4 nM. The MTD of 200 microg/kg total provides a dose level sufficient for anti-tumor activity in vitro and in a rodent model. Therefore, we propose that this agent is a promising drug for patients with surface CD3+ T cell malignancies.
Figures

References
-
- The Non-Hodgkin’s Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909–3918. - PubMed
-
- Suzushima H, Asou N, Nishimura S, Nishikawa K, Wang JX, Okubo T, Naito M, Hattori T, Takatsuki K. Double-negative (CD4- CD8-) T cells from adult T-cell leukemia patients also have poor expression of the T-cell receptor alpha beta/CD3 complex. Blood. 1993;81:1032–1039. - PubMed
-
- Sandberg Y, Almeida J, Gonzalez M, Lima M, Barcena P, Szczepanski T, van Gastel-Mol EJ, Wind H, Balanzategui A, van Dongen JJ, Miguel JF, Orfao A, Langerak AW. TCRgammadelta+ large granular lymphocyte leukemias reflect the spectrum of normal antigen-selected TCRgammadelta+ T-cells. Leukemia. 2006;20:505–513. doi: 10.1038/sj.leu.2404112. - DOI - PubMed
-
- Gentile TC, Uner AH, Hutchison RE, Wright J, Ben-Ezra J, Russell EC, Loughran TP., Jr CD3+, CD56+ aggressive variant of large granular lymphocyte leukemia. Blood. 1994;84:2315–2321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

