Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus
- PMID: 18258256
- PMCID: PMC2276640
- DOI: 10.1016/j.jmb.2008.01.031
Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus
Abstract
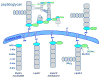
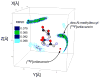
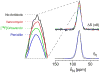
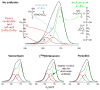
Solid-state NMR measurements performed on intact whole cells of Staphylococcus aureus labeled selectively in vivo have established that des-N-methylleucyl oritavancin (which has antimicrobial activity) binds to the cell-wall peptidoglycan, even though removal of the terminal N-methylleucyl residue destroys the D-Ala-D-Ala binding pocket. By contrast, the des-N-methylleucyl form of vancomycin (which has no antimicrobial activity) does not bind to the cell wall. Solid-state NMR has also determined that oritavancin and vancomycin are comparable inhibitors of transglycosylation, but that oritavancin is a more potent inhibitor of transpeptidation. This combination of effects on cell-wall binding and biosynthesis is interpreted in terms of a recent proposal that oritavancin-like glycopeptides have two cell-wall binding sites: the well-known peptidoglycan D-Ala-D-Ala pentapeptide stem terminus and the pentaglycyl bridging segment. The resulting dual mode of action provides a structural framework for coordinated cell-wall assembly that accounts for the enhanced potency of oritavancin and oritavancin-like analogues against vancomycin-resistant organisms.
Figures


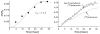




References
-
- Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–3. - PubMed
-
- CDC. Staphylococcus aureus resistant to vancomycin-United States, 2002. Morb Mortal Wkly Rep. 2002;51:565–7. - PubMed
-
- Noble WC, Virani Z, Cree RG. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;72:195–8. - PubMed
-
- Van Bambeke F. Glycopeptides in clinical development: pharmacological profile and clinical perspectives. Curr Opin Pharmacol. 2004;4:471–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

