Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2
- PMID: 18258597
- PMCID: PMC2431037
- DOI: 10.1074/jbc.M707247200
Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2
Abstract
There are several lines of evidence that the podocyte slit diaphragm (SD), which serves as a structural framework for the filtration barrier in kidney glomerulus, also plays an essential role as a signaling platform. Several SD components including nephrin and TRPC6 are known to be phosphorylated by a Src family tyrosine kinase (SFK), Fyn. Here we have characterized Neph1, another SD component, as a novel substrate of SFK. Fyn interacts with and phosphorylates the cytoplasmic domain of Neph1 in vitro and in intact cells. Peptide mass fingerprinting and site-directed mutagenesis identified several tyrosine phosphorylation sites. In pull-down assays using rat glomerular lysates, Neph1 but not nephrin specifically binds to adaptor protein Grb2 and tyrosine kinase Csk in a phosphorylation-dependent manner. Both tyrosine 637 and 638 of Neph1 are crucial for Neph1-Grb2 binding. Phosphorylation of tyrosine 637 is significantly up-regulated in in vivo models of podocyte injury. Furthermore, Neph1 attenuates ERK activation elicited by Fyn, and this inhibitory effect requires the intact binding motif for the Grb2 SH2 domain. Our results shown here demonstrate that Neph1 is a novel in vivo substrate of SFK and suggest that Neph1 modulates ERK signaling through phosphorylation-dependent interaction with Grb2. Thus, SFK orchestrates a wide spectrum of protein-protein interactions and intracellular signaling networks at SD through tyrosine phosphorylation.
Figures
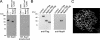



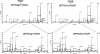




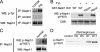
References
-
- Pavenstadt, H., Kriz, W., and Kretzler, M. (2003) Physiol. Rev. 83 253-307 - PubMed
-
- Kestila, M., Lenkkeri, U., Mannikko, M., Lamerdin, J., McCready, P., Putaala, H., Ruotsalainen, V., Morita, T., Nissinen, M., Herva, R., Kashtan, C. E., Peltonen, L., Holmberg, C., Olsen, A., and Tryggvason, K. (1998) Mol. Cell. 1 575-582 - PubMed
-
- Putaala, H., Soininen, R., Kilpelainen, P., Wartiovaara, J., and Tryggvason, K. (2001) Hum. Mol. Genet. 10 1-8 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

