The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice
- PMID: 18258755
- PMCID: PMC2862364
- DOI: 10.1177/0748730407311254
The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice
Abstract
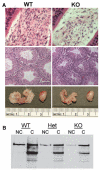
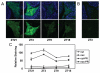
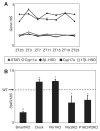
Although it is well established that the circadian clock regulates mammalian reproductive physiology, the molecular mechanisms by which this regulation occurs are not clear. The authors investigated the reproductive capacity of mice lacking Bmal1 (Arntl, Mop3), one of the central circadian clock genes. They found that both male and female Bmal1 knockout (KO) mice are infertile. Gross and microscopic inspection of the reproductive anatomy of both sexes suggested deficiencies in steroidogenesis. Male Bmal1 KO mice had low testosterone and high luteinizing hormone serum concentrations, suggesting a defect in testicular Leydig cells. Importantly, Leydig cells rhythmically express BMAL1 protein, suggesting peripheral control of testosterone production by this clock protein. Expression of steroidogenic genes was reduced in testes and other steroidogenic tissues of Bmal1 KO mice. In particular, expression of the steroidogenic acute regulatory protein (StAR) gene and protein, which regulates the rate-limiting step of steroidogenesis, was decreased in testes from Bmal1 KO mice. A direct effect of BMAL1 on StAR expression in Leydig cells was indicated by in vitro experiments showing enhancement of StAR transcription by BMAL1. Other hormonal defects in male Bmal1 KO mice suggest that BMAL1 also has functions in reproductive physiology outside of the testis. These results enhance understanding of how the circadian clock regulates reproduction.
Figures


References
-
- Alvarez JD, Chen D, Storer E, Sehgal A. Non-cyclic and developmental stage-specific expression of circadian clock proteins during murine spermatogenesis. Biol Reprod. 2003;69:81–91. - PubMed
-
- Alvarez JD, Sehgal A. The thymus is similar to the testis in its pattern of circadian clock gene expression. J Biol Rhythms. 2005;20:111–121. - PubMed
-
- Andrews WW, Ojeda SR. A detailed analysis of the serum luteinizing hormone secretory profile in conscious, free-moving female rats during the time of puberty. Endocrinology. 1981;109:2032–2039. - PubMed
-
- Apter D, Butzow T, Laughlin G, Yen S. Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: Pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab. 1993;76:940–949. - PubMed
-
- Ascoli M. Characterization of several clonal lines of cultured Leydig tumor cells: Gonadotropin receptors and steroidogenic responses. Endocrinology. 1981;108:88–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

