Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation
- PMID: 18258849
- PMCID: PMC2258256
- DOI: 10.2353/ajpath.2008.070286
Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation
Abstract
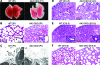
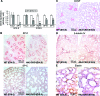
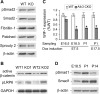
Bone morphogenetic proteins (BMPs) play important roles in regulating lung development and function although the endogenous regulatory effects of BMP signaling are still controversial. We found that BMP type I receptor Alk3 is expressed predominantly in airway epithelial cells during development. The function of Alk3 in lung development was determined using an inducible knockout mouse model by crossing epithelial cell-specific Cre transgenic mice SPC-rtTA/TetO-Cre and floxed-Alk3 mice. Abrogation of Alk3 in mouse lung epithelia from either early lung organogenesis or late gestation resulted in similar neonatal respiratory distress phenotypes accompanied by collapsed lungs. Early-induction of Alk3 knockout in lung epithelial cells caused retardation of early lung branching morphogenesis, reduced cell proliferation, and differentiation. However, late gestation induction of the knockout caused changes in cell proliferation and survival, as shown by altered cell biology, reduced expression of peripheral epithelial markers (Clara cell-specific protein, surfactant protein C, and aquaporin-5), and lack of surfactant secretion. Furthermore, canonical Wnt signaling was perturbed, possibly through reduced Wnt inhibitory factor-1 expression in Alk3-knockout lungs. Therefore, our data suggest that deficiency of appropriate BMP signaling in lung epithelial cells results in prenatal lung malformation, neonatal atelectasis, and respiratory failure.
Figures



References
-
- Warburton D, Gauldie J, Bellusci S, Shi W. Lung development and susceptibility to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:668–672. - PubMed
-
- Ten Have-Opbroek AA. Lung development in the mouse embryo. Exp Lung Res. 1991;17:111–130. - PubMed
-
- Hilfer SR. Morphogenesis of the lung: control of embryonic and fetal branching. Annu Rev Physiol. 1996;58:93–113. - PubMed
-
- Hogan BL. Morphogenesis. Cell. 1999;96:225–233. - PubMed
-
- Hogan BL, Grindley J, Bellusci S, Dunn NR, Emoto H, Itoh N. Branching morphogenesis of the lung: new models for a classical problem. Cold Spring Harb Symp Quant Biol. 1997;62:249–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

