c-kit is required for cardiomyocyte terminal differentiation
- PMID: 18258857
- PMCID: PMC2763373
- DOI: 10.1161/CIRCRESAHA.107.161737
c-kit is required for cardiomyocyte terminal differentiation
Abstract
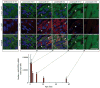
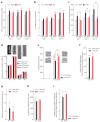
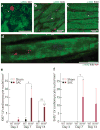
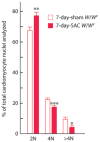
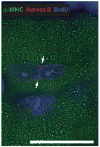
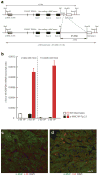
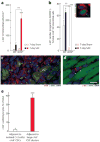
c-kit, the transmembrane tyrosine kinase receptor for stem cell factor, is required for melanocyte and mast cell development, hematopoiesis, and differentiation of spermatogonial stem cells. We show here that in the heart, c-kit is expressed not only by cardiac stem cells but also by cardiomyocytes, commencing immediately after birth and terminating a few days later, coincident with the onset of cardiomyocyte terminal differentiation. To examine the function of c-kit in cardiomyocyte terminal differentiation, we used compound heterozygous mice carrying the W (null) and W(v) (dominant negative) mutations of c-kit. In vivo, adult W/W(v) cardiomyocytes are phenotypically indistinguishable from their wild-type counterparts. After acute pressure overload adult W/W(v) cardiomyocytes reenter the cell cycle and proliferate, leading to left ventricular growth; furthermore in transgenic mice with cardiomyocyte-restricted overexpression of the dominant negative W(v) mutant, pressure overload causes cardiomyocytes to reenter the cell cycle. In contrast, in wild-type mice left ventricular growth after pressure overload results mainly from cardiomyocyte hypertrophy. Importantly, W/W(v) mice with pressure overload-induced cardiomyocyte hyperplasia had improved left ventricular function and survival. In W/W(v) mice, c-kit dysfunction also resulted in an approximately 14-fold decrease (P<0.01) in the number of c-kit(+)/GATA4(+) cardiac progenitors. These findings identify novel functions for c-kit: promotion of cardiac stem cell differentiation and regulation of cardiomyocyte terminal differentiation.
Figures

References
-
- Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. - PubMed
-
- MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–313. - PubMed
-
- Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

