Sustained activation and tumor targeting of NKT cells using a CD1d-anti-HER2-scFv fusion protein induce antitumor effects in mice
- PMID: 18259610
- PMCID: PMC2230658
- DOI: 10.1172/JCI33249
Sustained activation and tumor targeting of NKT cells using a CD1d-anti-HER2-scFv fusion protein induce antitumor effects in mice
Abstract
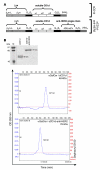
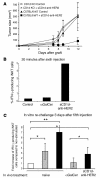
Invariant NKT (iNKT) cells are potent activators of DCs, NK cells, and T cells, and their antitumor activity has been well demonstrated. A single injection of the high-affinity CD1d ligand alpha-galactosylceramide (alphaGalCer) leads to short-lived iNKT cell activation followed, however, by long-term anergy, limiting its therapeutic use. In contrast, we demonstrated here that when alphaGalCer was loaded on a recombinant soluble CD1d molecule (alphaGalCer/sCD1d), repeated injections led to sustained iNKT and NK cell activation associated with IFN-gamma secretion as well as DC maturation in mice. Most importantly, when alphaGalCer/sCD1d was fused to a HER2-specific scFv antibody fragment, potent inhibition of experimental lung metastasis and established s.c. tumors was obtained when systemic treatment was started 2-7 days after the injection of HER2-expressing B16 melanoma cells. In contrast, administration of free alphaGalCer at this time had no effect. The antitumor activity of the CD1d-anti-HER2 fusion protein was associated with HER2-specific tumor localization and accumulation of iNKT, NK, and T cells at the tumor site. Targeting iNKT cells to the tumor site thus may activate a combined innate and adaptive immune response that may prove to be effective in cancer immunotherapy.
Figures






References
-
- de Visser K.E., Eichten A., Coussens L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6:24–37. - PubMed
-
- Dunn G.P., Old L.J., Schreiber R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. - PubMed
-
- Boon T., et al. Human T cell responses against melanoma. Annu. Rev. Immunol. 2006;24:175–208. - PubMed
-
- Michaeli D. Vaccines and monoclonal antibodies. Semin. Oncol. 2005;32:S82–S86. - PubMed
-
- Bendelac A., et al. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

