Synthesis of ethyl (1S,2R,3S,4S,5S)-2,3-O-(isopropylidene)-4-hydroxy-bicyclo[3.1.0]hexane-carboxylate from L-ribose: a versatile chiral synthon for preparation of adenosine and P2 receptor ligands
- PMID: 18260011
- PMCID: PMC6943831
- DOI: 10.1080/15257770701845253
Synthesis of ethyl (1S,2R,3S,4S,5S)-2,3-O-(isopropylidene)-4-hydroxy-bicyclo[3.1.0]hexane-carboxylate from L-ribose: a versatile chiral synthon for preparation of adenosine and P2 receptor ligands
Abstract
Substitution of the ribose moiety of various nucleosides and nucleotides with the (N)-methanocarba ring system increases the potency and selectivity as ligands at certain subtypes of adenosine and P2 receptors. We have prepared a key intermediate in the synthesis of these derivatives, ethyl (1S,2R,3S,4S,5S)-2,3-O-(isopropylidene)-4-hydroxybicyclo[3.1.0]hexane-carboxylate (15), starting from L-ribose (8) as a readily available, enantiopure building block. L-ribose was converted to the corresponding 5'-iodo derivative (9), which was cleaved reductively with Zn. Improvements were made in subsequent steps corresponding to a published route to biologically important (N)-methanocarba 5'-uronamido nucleosides, and new steps were added to prepare related 5'-nucleotides.
Figures
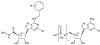



Similar articles
-
Selective A(3) adenosine receptor antagonists derived from nucleosides containing a bicyclo[3.1.0]hexane ring system.Bioorg Med Chem. 2008 Sep 15;16(18):8546-56. doi: 10.1016/j.bmc.2008.08.007. Epub 2008 Aug 7. Bioorg Med Chem. 2008. PMID: 18752961 Free PMC article.
-
Ribose modified nucleosides and nucleotides as ligands for purine receptors.Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):333-41. doi: 10.1081/NCN-100002305. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11563046 Free PMC article. Review.
-
2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists.J Med Chem. 2003 Nov 6;46(23):4974-87. doi: 10.1021/jm030127+. J Med Chem. 2003. PMID: 14584948 Free PMC article.
-
Conformationally locked nucleoside analogues. Synthesis of dideoxycarbocyclic nucleoside analogues structurally related to neplanocin C.J Med Chem. 1994 Sep 30;37(20):3389-99. doi: 10.1021/jm00046a024. J Med Chem. 1994. PMID: 7932567
-
Action of nucleosides and nucleotides at 7 transmembrane-spanning receptors.Nucleosides Nucleotides Nucleic Acids. 2006;25(12):1425-36. doi: 10.1080/15257770600919027. Nucleosides Nucleotides Nucleic Acids. 2006. PMID: 17067963 Free PMC article. Review.
Cited by
-
Molecular recognition in the P2Y(14) receptor: Probing the structurally permissive terminal sugar moiety of uridine-5'-diphosphoglucose.Bioorg Med Chem. 2009 Jul 15;17(14):5298-311. doi: 10.1016/j.bmc.2009.05.024. Epub 2009 May 15. Bioorg Med Chem. 2009. PMID: 19502066 Free PMC article.
-
Structure-activity relationship of (N)-Methanocarba phosphonate analogues of 5'-AMP as cardioprotective agents acting through a cardiac P2X receptor.J Med Chem. 2010 Mar 25;53(6):2562-76. doi: 10.1021/jm9018542. J Med Chem. 2010. PMID: 20192270 Free PMC article.
-
Truncated Nucleosides as A(3) Adenosine Receptor Ligands: Combined 2-Arylethynyl and Bicyclohexane Substitutions.ACS Med Chem Lett. 2012 Jul 12;3(7):596-601. doi: 10.1021/ml300107e. Epub 2012 Jun 11. ACS Med Chem Lett. 2012. PMID: 23145215 Free PMC article.
-
5'-Phosphate and 5'-phosphonate ester derivatives of (N)-methanocarba adenosine with in vivo cardioprotective activity.J Med Chem. 2013 Feb 14;56(3):902-14. doi: 10.1021/jm301372c. Epub 2013 Jan 22. J Med Chem. 2013. PMID: 23286881 Free PMC article.
-
Structure-Based Design, Synthesis by Click Chemistry and in Vivo Activity of Highly Selective A3 Adenosine Receptor Agonists.Medchemcomm. 2015;6:555-563. doi: 10.1039/C4MD00571F. Medchemcomm. 2015. PMID: 26236460 Free PMC article.
References
-
- Altona C; Sundaralingham M Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J. Am. Chem. Soc 1972, 94, 8205–8212. - PubMed
-
- Marquez VE; Siddiqui MA; Ezzitouni A; Russ P; Wang J; Wagner RW; Matteucci MD Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J. Med. Chem 1996, 39, 3739–3747. - PubMed
-
- Kim HS; Ravi RG; Marquez VE; Maddileti S; Wihlborg A-K; Erlinge D; Malmsjö M; Boyer JL; Harden TK; Jacobson KA Methanocarba modification of uracil and adenine nucleotides: High potency of Northern ring conformation at P2Y1, P2Y2, P2Y4 and P2Y11, but not P2Y6 receptors. J. Med. Chem 2002, 45, 208–218. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
