Sequences in the UL11 tegument protein of herpes simplex virus that control association with detergent-resistant membranes
- PMID: 18261757
- PMCID: PMC2409213
- DOI: 10.1016/j.virol.2008.01.007
Sequences in the UL11 tegument protein of herpes simplex virus that control association with detergent-resistant membranes
Abstract
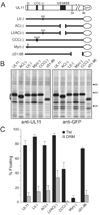
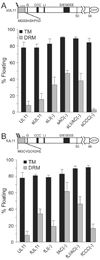
The product of the UL11 gene of HSV-1 is a small, membrane-bound tegument protein with features that are conserved among all herpesviruses. For all viruses examined, mutants lacking this protein (or its homolog) have budding defects and accumulate capsids in the cytoplasm of the infected cell. UL11 binds to the cytoplasmic faces of host membranes via N-terminal myristate and nearby palmitate moieties. These fatty-acid modifications are typical of proteins that localize to detergent-resistant membranes (DRMs), and the experiments described here revealed that a small amount (approximately 10%) of UL11 retains the ability to float in sucrose gradients following treatment of cells with Triton X-100. However, mutants lacking sequences previously shown to be involved in the trafficking of UL11 from the plasma membrane (LI and acidic cluster motifs) were found to have a dramatically increased association with DRMs. These findings emphasize the dynamic properties of this poorly-understood but conserved tegument protein.
Figures
References
-
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 1994;269(24):16701–16705. - PubMed
-
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296(5574):1821–1825. - PubMed
-
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

