Dissolving microneedles for transdermal drug delivery
- PMID: 18261792
- PMCID: PMC2293299
- DOI: 10.1016/j.biomaterials.2007.12.048
Dissolving microneedles for transdermal drug delivery
Abstract
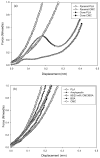
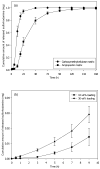
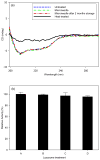
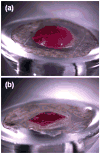
Microfabrication technology has been adapted to produce micron-scale needles as a safer and painless alternative to hypodermic needle injection, especially for protein biotherapeutics and vaccines. This study presents a design that encapsulates molecules within microneedles that dissolve within the skin for bolus or sustained delivery and leave behind no biohazardous sharp medical waste. A fabrication process was developed based on casting a viscous aqueous solution during centrifugation to fill a micro-fabricated mold with biocompatible carboxymethylcellulose or amylopectin formulations. This process encapsulated sulforhodamine B, bovine serum albumin, and lysozyme; lysozyme was shown to retain full enzymatic activity after encapsulation and to remain 96% active after storage for 2 months at room temperature. Microneedles were also shown to be strong enough to insert into cadaver skin and then to dissolve within minutes. Bolus delivery was achieved by encapsulating molecules just within microneedle shafts. For the first time, sustained delivery over hours to days was achieved by encapsulating molecules within the microneedle backing, which served as a controlled release reservoir that delivered molecules by a combination of swelling the backing with interstitial fluid drawn out of the skin and molecule diffusion into the skin via channels formed by dissolved microneedles. We conclude that dissolving microneedles can be designed to gently encapsulate molecules, insert into skin, and enable bolus or sustained release delivery.
Figures





References
-
- Daugherty AL, Mrsny RJ. Emerging technologies that overcome biological barriers for therapeutic protein delivery. Expert Opinion on Biological Therapy. 2003;3(7):1071–1081. - PubMed
-
- Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: Prevalence and associations. American Journal of Tropical Medicine and Hygiene. 2003;68(3):341–344. - PubMed
-
- Reed ML, Lye WK. Microsystems for drug and gene delivery. Proceedings of the IEEE. 2004;92(1):56–75.
-
- Prausnitz MR. Microneedles for transdermal drug delivery. Advanced Drug Delivery Reviews. 2004;56(5):581–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

