FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells
- PMID: 18263593
- PMCID: PMC2902396
- DOI: 10.1093/carcin/bgn026
FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells
Abstract
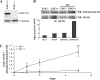
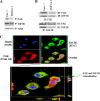
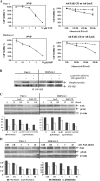
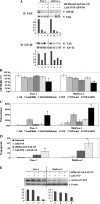
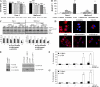
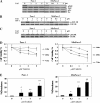
Pancreatic cancer is a lethal disease accounting for the fourth leading cause of cancer death in USA. Focal adhesion kinase (FAK) and the insulin-like growth factor-I receptor (IGF-1R) are tyrosine kinases that activate common pathways, leading to increased proliferation and cell survival. Sparse information is available regarding their contribution to the malignant behavior of pancreatic cancer. We analyzed the relationship between FAK and IGF-1R in human pancreatic cancer cells, determined which downstream signaling pathways are altered following kinase inhibition or downregulation and studied whether dual kinase inhibition represents a potential novel treatment strategy in this deadly disease. Using immunoprecipitation and confocal microscopy, we show for the first time that FAK and IGF-1R physically interact in pancreatic cancer cells and that inhibition of tyrosine phosphorylation of either kinase disrupts their interaction. Decreasing phosphorylation of either FAK or IGF-1R alone resulted in little inhibition of cell viability or increased apoptosis. However, dual inhibition of FAK, using either a dominant-negative construct (FAK-CD) or small interfering RNA, and IGF-1R, using a specific small molecule tyrosine kinase inhibitor (AEW-541) or stable expression of a truncated, mutated IGF-1R, led to a synergistic decrease in cell proliferation and phosphorylation of extracellular signal-regulated kinase (ERK) and increase in cell detachment and apoptosis compared with inhibition of either pathway alone. Dual kinase inhibition with FAK-CD and AEW-541 resulted in a marked increase in apoptosis when FAK was displaced from the focal adhesions. Inhibition of both tyrosine kinase activities via a novel single small molecular inhibitor (TAE 226), at low doses specific for FAK and IGF-1R, resulted in significant inhibition of cell viability, decrease in phosphorylation of ERK and Akt and increase in apoptosis accompanied by cleavage of Poly (ADP-ribose) polymerase (PARP) and activation of caspase-3 in pancreatic cancer cells. Thus, simultaneous inhibition of both tyrosine kinases represents a potential novel therapeutic approach in human pancreatic adenocarcinoma.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

