Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial
- PMID: 18263663
- PMCID: PMC2569194
- DOI: 10.1136/thx.2007.090647
Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial
Abstract
Background: Varenicline, a new treatment for smoking cessation, has demonstrated significantly greater efficacy over placebo and sustained release bupropion (bupropion SR). A study was undertaken to compare a 12-week standard regimen of varenicline with a 10-week standard regimen of transdermal nicotine replacement therapy (NRT) for smoking cessation.
Methods: In this 52-week, open-label, randomised, multicentre, phase 3 trial conducted in Belgium, France, The Netherlands, UK and USA, participants were randomly assigned (1:1) to receive varenicline uptitrated to 1 mg twice daily for 12 weeks or transdermal NRT (21 mg/day reducing to 7 mg/day) for 10 weeks. Non-treatment follow-up continued to week 52. The primary outcome was the biochemically confirmed (exhaled carbon monoxide < or = 10 ppm) self-reported continuous abstinence rate (CAR) for the last 4 weeks of the treatment period in participants who had taken at least one dose of treatment. Secondary outcomes included CAR from the last 4 weeks of treatment through weeks 24 and 52, and measures of craving, withdrawal and smoking satisfaction.
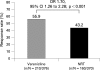
Results: A total of 376 and 370 participants assigned to varenicline and NRT, respectively, were eligible for analysis. The CAR for the last 4 weeks of treatment was significantly greater for varenicline (55.9%) than NRT (43.2%; OR 1.70, 95% CI 1.26 to 2.28, p<0.001). The week 52 CAR (NRT, weeks 8-52; varenicline, weeks 9-52) was 26.1% for varenicline and 20.3% for NRT (OR 1.40, 95% CI 0.99 to 1.99, p = 0.056). Varenicline significantly reduced craving (p<0.001), withdrawal symptoms (p<0.001) and smoking satisfaction (p<0.001) compared with NRT. The most frequent adverse event was nausea (varenicline, 37.2%; NRT, 9.7%).
Conclusions: The outcomes of this trial established that abstinence from smoking was greater and craving, withdrawal symptoms and smoking satisfaction were less at the end of treatment with varenicline than with transdermal NRT.
Trial registration number: NCT00143325.
Conflict of interest statement
Figures


Comment in
-
The place of varenicline in smoking cessation treatment.Thorax. 2008 Aug;63(8):666-8. doi: 10.1136/thx.2008.096081. Thorax. 2008. PMID: 18663065 No abstract available.
-
Pre-cessation varenicline treatment vs post-cessation NRT: an uneven playing field.Thorax. 2008 Aug;63(8):751-2; author reply 752. Thorax. 2008. PMID: 18663077 No abstract available.
-
Clinically significant outcomes in smoking cessation.Thorax. 2008 Aug;63(8):752; author reply 752-3. Thorax. 2008. PMID: 18663078 No abstract available.
References
-
- Fiore M. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guidelines. Respir Care 2000;45:1196–9 - PubMed
-
- Centers for Disease Control and Prevention Cigarette smoking among adults – United States, 2000. MMWR Morb Mortal Wkly Rep 2002;51:642–5 - PubMed
-
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99:29–38 - PubMed
-
- Tønnesen P, Carrozzi L, Fagerström K, et al. Smoking cessation in patients with respiratory diseases: a high priority, integral component of therapy. Eur Respir J 2007;29:390–417 - PubMed
-
- US Food and Drug Administration Electric orange book: approved drug products with therapeutic equivalence evaluations. 2007. http://www.fda.gov/cder/ob/default.htm (accessed 24 January 2008)
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
