Occludin independently regulates permeability under hydrostatic pressure and cell division in retinal pigment epithelial cells
- PMID: 18263810
- PMCID: PMC2483238
- DOI: 10.1167/iovs.07-1204
Occludin independently regulates permeability under hydrostatic pressure and cell division in retinal pigment epithelial cells
Abstract
Purpose: The aim of this study was to determine the function of the tight junction protein occludin in the control of permeability, under diffusive and hydrostatic pressures, and its contribution to the control of cell division in retinal pigment epithelium.
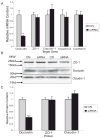
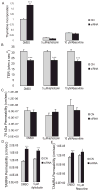
Methods: Occludin expression was inhibited in the human retinal pigment epithelial cell line ARPE-19 by siRNA. Depletion of occludin was confirmed by Western blot, confocal microscopy, and RT-PCR. Paracellular permeability of cell monolayers to fluorescently labeled 70 kDa dextran, 10 kDa dextran, and 467 Da tetramethylrhodamine (TAMRA) was examined under diffusive conditions or after the application of 10 cm H2O transmural pressure. Cell division rates were determined by tritiated thymidine incorporation and Ki67 immunoreactivity. Cell cycle inhibitors were used to determine whether changes in cell division affected permeability.
Results: Occludin depletion increased diffusive paracellular permeability to 467 Da TAMRA by 15%, and permeability under hydrostatic pressure was increased 50% compared with control. Conversely, depletion of occludin protein with siRNA did not alter diffusive permeability to 70 kDa and 10 kDa RITC-dextran, and permeability to 70 kDa dextran was twofold lower in occludin-depleted cells under hydrostatic pressure conditions. Occludin depletion also increased thymidine incorporation by 90% and Ki67-positive cells by 50%. Finally, cell cycle inhibitors did not alter the effect of occludin siRNA on paracellular permeability.
Conclusions: The data suggest that occludin regulates tight junction permeability in response to changes in hydrostatic pressure. Furthermore, these data suggest that occludin also contributes to the control of cell division, demonstrating a novel function for this tight junction protein.
Figures




Similar articles
-
Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function.Invest Ophthalmol Vis Sci. 2007 Sep;48(9):3928-36. doi: 10.1167/iovs.06-1536. Invest Ophthalmol Vis Sci. 2007. PMID: 17724169 Free PMC article.
-
Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells.Exp Eye Res. 2009 Dec;89(6):913-20. doi: 10.1016/j.exer.2009.07.017. Epub 2009 Aug 4. Exp Eye Res. 2009. PMID: 19660451
-
Oxidative stress affects the junctional integrity of retinal pigment epithelial cells.Invest Ophthalmol Vis Sci. 2004 Feb;45(2):675-84. doi: 10.1167/iovs.03-0351. Invest Ophthalmol Vis Sci. 2004. PMID: 14744914
-
Occludin and the functions of tight junctions.Int Rev Cytol. 1999;186:117-46. doi: 10.1016/s0074-7696(08)61052-9. Int Rev Cytol. 1999. PMID: 9770298 Review.
-
Tight junctions and the molecular basis for regulation of paracellular permeability.Am J Physiol. 1995 Oct;269(4 Pt 1):G467-75. doi: 10.1152/ajpgi.1995.269.4.G467. Am J Physiol. 1995. PMID: 7485497 Review.
Cited by
-
Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability.J Biol Chem. 2009 Jul 31;284(31):21036-46. doi: 10.1074/jbc.M109.016766. Epub 2009 May 28. J Biol Chem. 2009. PMID: 19478092 Free PMC article.
-
Glucocorticoid induction of occludin expression and endothelial barrier requires transcription factor p54 NONO.Invest Ophthalmol Vis Sci. 2013 Jun 12;54(6):4007-15. doi: 10.1167/iovs.13-11980. Invest Ophthalmol Vis Sci. 2013. PMID: 23640037 Free PMC article.
-
Meprin A impairs epithelial barrier function, enhances monocyte migration, and cleaves the tight junction protein occludin.Am J Physiol Renal Physiol. 2013 Sep 1;305(5):F714-26. doi: 10.1152/ajprenal.00179.2012. Epub 2013 Jun 26. Am J Physiol Renal Physiol. 2013. PMID: 23804454 Free PMC article.
-
Knockdown of sodium-calcium exchanger 1 induces epithelial-to-mesenchymal transition in kidney epithelial cells.J Biol Chem. 2017 Jul 7;292(27):11388-11399. doi: 10.1074/jbc.M116.752352. Epub 2017 May 26. J Biol Chem. 2017. PMID: 28550085 Free PMC article.
-
Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction.Biochem J. 2012 Sep 15;446(3):455-67. doi: 10.1042/BJ20111961. Biochem J. 2012. PMID: 22721706 Free PMC article.
References
-
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1– 44. - PubMed
-
- Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev. 2005;57:815– 855. - PubMed
-
- Marmor MF, Wolfensberger TJ. The Retinal Pigment Epithelium: Function and Disease. New York: Oxford University Press; 1998.
-
- Korte GE, Burns MS, Bellhorn RW. Epithelium-capillary interactions in the eye: the retinal pigment epithelium and the choriocapillaris. Int Rev Cytol. 1989;114:221–248. - PubMed
-
- Vinores SA, Kuchle M, Derevjanik NL, et al. Blood-retinal barrier breakdown in retinitis pigmentosa: light and electron microscopic immunolocalization. Histol Histopathol. 1995;10:913–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

