Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain
- PMID: 18264108
- PMCID: PMC2279180
- DOI: 10.1038/nm1723
Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain
Abstract
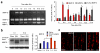
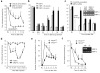
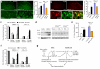
Treatment of neuropathic pain, triggered by multiple insults to the nervous system, is a clinical challenge because the underlying mechanisms of neuropathic pain development remain poorly understood. Most treatments do not differentiate between different phases of neuropathic pain pathophysiology and simply focus on blocking neurotransmission, producing transient pain relief. Here, we report that early- and late-phase neuropathic pain development in rats and mice after nerve injury require different matrix metalloproteinases (MMPs). After spinal nerve ligation, MMP-9 shows a rapid and transient upregulation in injured dorsal root ganglion (DRG) primary sensory neurons consistent with an early phase of neuropathic pain, whereas MMP-2 shows a delayed response in DRG satellite cells and spinal astrocytes consistent with a late phase of neuropathic pain. Local inhibition of MMP-9 by an intrathecal route inhibits the early phase of neuropathic pain, whereas inhibition of MMP-2 suppresses the late phase of neuropathic pain. Further, intrathecal administration of MMP-9 or MMP-2 is sufficient to produce neuropathic pain symptoms. After nerve injury, MMP-9 induces neuropathic pain through interleukin-1beta cleavage and microglial activation at early times, whereas MMP-2 maintains neuropathic pain through interleukin-1beta cleavage and astrocyte activation at later times. Inhibition of MMP may provide a novel therapeutic approach for the treatment of neuropathic pain at different phases.
Figures

Comment in
-
Taking two cuts at pain.Nat Med. 2008 Mar;14(3):243-4. doi: 10.1038/nm0308-243. Nat Med. 2008. PMID: 18323841 No abstract available.
Similar articles
-
p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain.J Neurosci. 2003 May 15;23(10):4017-22. doi: 10.1523/JNEUROSCI.23-10-04017.2003. J Neurosci. 2003. PMID: 12764087 Free PMC article.
-
Intrathecal CGS-26303 Pretreatment Attenuates Spinal Nerve Ligation-Induced Neuropathic Pain in the Spinal Cord.World Neurosurg. 2016 Jul;91:532-541.e1. doi: 10.1016/j.wneu.2016.02.093. Epub 2016 Mar 4. World Neurosurg. 2016. PMID: 26947729
-
ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model.Pain. 2005 Mar;114(1-2):149-59. doi: 10.1016/j.pain.2004.12.022. Epub 2005 Jan 26. Pain. 2005. PMID: 15733640
-
Matrix metalloprotease regulation of neuropathic pain.Trends Pharmacol Sci. 2009 Jul;30(7):336-40. doi: 10.1016/j.tips.2009.04.002. Epub 2009 Jun 10. Trends Pharmacol Sci. 2009. PMID: 19523695 Free PMC article.
-
Matrix metalloproteinases: potential therapeutic target for diabetic neuropathic pain.Expert Opin Ther Targets. 2015 Feb;19(2):177-85. doi: 10.1517/14728222.2014.960844. Epub 2014 Sep 22. Expert Opin Ther Targets. 2015. PMID: 25243524 Review.
Cited by
-
Chemogenetic Regulation of CX3CR1-Expressing Microglia Using Gi-DREADD Exerts Sex-Dependent Anti-Allodynic Effects in Mouse Models of Neuropathic Pain.Front Pharmacol. 2020 Jun 19;11:925. doi: 10.3389/fphar.2020.00925. eCollection 2020. Front Pharmacol. 2020. PMID: 32636748 Free PMC article.
-
Analgesic mechanism of electroacupuncture in a rat L5 spinal nerve ligation model.Exp Ther Med. 2015 Mar;9(3):987-991. doi: 10.3892/etm.2015.2165. Epub 2015 Jan 2. Exp Ther Med. 2015. PMID: 25667665 Free PMC article.
-
Microglia: sculptors of neuropathic pain?R Soc Open Sci. 2020 Jun 17;7(6):200260. doi: 10.1098/rsos.200260. eCollection 2020 Jun. R Soc Open Sci. 2020. PMID: 32742693 Free PMC article. Review.
-
Tissue plasminogen activator contributes to morphine tolerance and induces mechanical allodynia via astrocytic IL-1β and ERK signaling in the spinal cord of mice.Neuroscience. 2013 Sep 5;247:376-85. doi: 10.1016/j.neuroscience.2013.05.018. Epub 2013 May 21. Neuroscience. 2013. PMID: 23707980 Free PMC article.
-
Review of the Mechanisms of Snake Venom Induced Pain: It's All about Location, Location, Location.Int J Mol Sci. 2022 Feb 15;23(4):2128. doi: 10.3390/ijms23042128. Int J Mol Sci. 2022. PMID: 35216244 Free PMC article. Review.
References
-
- Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci.STKE. 2004;2004:reE14. - PubMed
-
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. - PubMed
-
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. - PubMed
-
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. - PubMed
-
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat.Rev.Immunol. 2004;4:617–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054932/NS/NINDS NIH HHS/United States
- R01 NS048422/NS/NINDS NIH HHS/United States
- R01-NS48422/NS/NINDS NIH HHS/United States
- R01-NS37074/NS/NINDS NIH HHS/United States
- R01 NS035884/NS/NINDS NIH HHS/United States
- R03 TW007180/TW/FIC NIH HHS/United States
- R01 NS037074/NS/NINDS NIH HHS/United States
- P50 NS010828/NS/NINDS NIH HHS/United States
- R01-NS54362/NS/NINDS NIH HHS/United States
- P01 NS055104/NS/NINDS NIH HHS/United States
- P01-NS55104/NS/NINDS NIH HHS/United States
- R01 NS056458/NS/NINDS NIH HHS/United States
- R01 NS093415/NS/NINDS NIH HHS/United States
- R01-NS56458/NS/NINDS NIH HHS/United States
- R01-DE17794/DE/NIDCR NIH HHS/United States
- TW7180/TW/FIC NIH HHS/United States
- F30 NS054362/NS/NINDS NIH HHS/United States
- P50-NS10828/NS/NINDS NIH HHS/United States
- R01 DE017794/DE/NIDCR NIH HHS/United States
- R37 NS037074/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

