Enhanced nitric oxide and cyclic GMP formation plays a role in the anti-platelet activity of simvastatin
- PMID: 18264124
- PMCID: PMC2275444
- DOI: 10.1038/bjp.2008.19
Enhanced nitric oxide and cyclic GMP formation plays a role in the anti-platelet activity of simvastatin
Abstract
Background and purpose: It has been found that 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) exert various vascular protective effects, beyond their cholesterol-lowering property, including inhibition of platelet-dependent thrombus formation. The objective of the present study was to determine whether the nitric oxide (NO)/cyclic GMP-mediated processes in platelets contribute to the anti-aggregatory activity of simvastatin.
Experimental approach: After rabbit platelets were incubated with simvastatin for 5 min, aggregation was induced and the platelet aggregation, nitric oxide synthase activity, guanylyl cyclase activity, NO and cyclic GMP formation were measured appropriately.
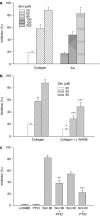
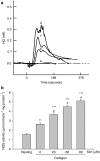
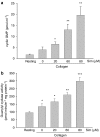
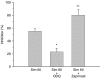
Key results: Treatment with simvastatin concentration-dependently inhibited platelet aggregation induced by collagen or arachidonic acid with an IC(50) range of 52-158 microM. We also demonstrated that simvastatin (20-80 microM) concentration-dependently further enhanced collagen-induced NO and cyclic GMP formation through increasing NOS activity (from 2.64+/-0.12 to 3.52+/-0.21-5.10+/-0.14 micromol min(-1) mg protein(-1)) and guanylyl cyclase activity (from 142.9+/-7.2 to 163.5+/-17.5-283.8+/-19.5 pmol min(-1) mg protein(-1)) in the platelets. On the contrary, inhibition of platelet aggregation by simvastatin was markedly attenuated (by about 50%) by addition of a nitric oxide synthase inhibitor, a NO scavenger or a NO-sensitive guanylyl cyclase inhibitor. The anti-aggregatory effects of simvastatin were significantly increased by addition of a selective inhibitor of cyclic GMP phosphodiesterase.
Conclusions and implications: Our findings indicate that enhancement of a NO/cyclic GMP-mediated process plays an important role in the anti-aggregatory activity of simvastatin.
Figures
References
-
- Alvarez de S, Andriantsitohaina R. Simvastatin and Ca2+ signaling in endothelial cells: involvement of Rho protein. Biochem Biophys Res Commun. 2001;280:486–490. - PubMed
-
- Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114:447–453. - PubMed
-
- Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering-are they clinically relevant. Eur Heart J. 2003;24:225–248. - PubMed
-
- Broijersen A, Eriksson M, Leijd B, Angelin B, Hjemdahl P. No influence of simvastatin treatment on platelet function in vivo in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:273–278. - PubMed
-
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, et al. c-AMP and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

