GABA(B) receptors in neuroendocrine regulation
- PMID: 18264754
- PMCID: PMC11515035
- DOI: 10.1007/s10571-008-9263-4
GABA(B) receptors in neuroendocrine regulation
Abstract
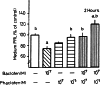
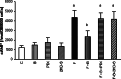
Gamma-amino butyric acid (GABA), in addition to being a metabolic intermediate and the main inhibitory neurotransmitter in the synaptic cleft, is postulated as a neurohormone, a paracrine signaling molecule, and a trophic factor. It acts through pre- and post-synaptic receptors, named GABA(A) and GABA(C) (ionotropic receptors) and GABA(B) (metabotropic receptor). Here we reviewed the participation of GABA(B) receptors in the regulation of the hypothalamic-pituitary-gonadal axis, using physiological, biochemical, and pharmacological approaches in rats, as well as in GABA(B1) knock-out mice, that lack functional GABA(B) receptors. Our general conclusion indicates that GABA(B )receptors participate in the regulation of pituitary hormone secretion acting both in the central nervous system and directly on the gland. PRL and gonadotropin axes are affected by GABA(B) receptor activation, as demonstrated in the rat and also in the GABA(B1) knock-out mouse. In addition, hypothalamic and pituitary GABA(B) receptor expression is modulated by steroid hormones. GABA participation in the brain control of pituitary secretion through GABA(B) receptors depends on physiological conditions, being age and sex critical factors.These results indicate that patients receiving GABA(B) agonists/antagonists should be monitored for possible endocrine side effects.
Figures




References
-
- Anderson R, Mitchell R (1986a) Biphasic effect of GABAA receptor agonists on prolactin secretion: evidence for two types of GABAA receptor complex on lactotroprs. Eur J Pharmacol 124:1–9 - PubMed
-
- Anderson R, Mitchell R (1986b) Effects of gamma-aminobutiric acid receptor agonist on the secretion of growth hormone, luteinizing hormone, adrenocorticotrophic hormone and thyroid-stimulating hormone from the rat pituitary gland in vitro. J Endocrinol 108:1–8 - PubMed
-
- Apud JA, Cocchi D, Locatelli V, Masotto C, Muller EE, Racagni G (1989) Biochemical and functional aspects on the control of prolactin release by the hypothalamo-pituitary GABAergic system. Psyconeuroendocrinol 14:3–17 - PubMed
-
- Arakelian MC, Libertun C (1977) H1 and H2 histamine receptor participation in the brain control of prolactin secretion in lactacting rats. Endocrinology 100:890–895 - PubMed
-
- Arakelian MC, Foglia VG, Libertun C (1984) Prolactin and milk ejection during the first 20 minutes of suckling in the rat: blockade by nembutal and by amino oxyacetic acid. Horm Metab Res 16:154 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

