Hydrophilic interaction liquid chromatography (HILIC) in proteomics
- PMID: 18264818
- PMCID: PMC2324128
- DOI: 10.1007/s00216-008-1865-7
Hydrophilic interaction liquid chromatography (HILIC) in proteomics
Abstract
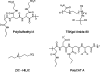
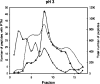
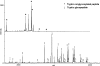
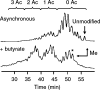
In proteomics, nanoflow multidimensional chromatography is now the gold standard for the separation of complex mixtures of peptides as generated by in-solution digestion of whole-cell lysates. Ideally, the different stationary phases used in multidimensional chromatography should provide orthogonal separation characteristics. For this reason, the combination of strong cation exchange chromatography (SCX) and reversed-phase (RP) chromatography is the most widely used combination for the separation of peptides. Here, we review the potential of hydrophilic interaction liquid chromatography (HILIC) as a separation tool in the multidimensional separation of peptides in proteomics applications. Recent work has revealed that HILIC may provide an excellent alternative to SCX, possessing several advantages in the area of separation power and targeted analysis of protein post-translational modifications. [figure: see text]
Figures


Similar articles
-
Evaluation and optimization of ZIC-HILIC-RP as an alternative MudPIT strategy.J Proteome Res. 2007 Mar;6(3):937-46. doi: 10.1021/pr060589m. Epub 2007 Jan 27. J Proteome Res. 2007. PMID: 17256977
-
Online coupling of hydrophilic interaction/strong cation exchange/reversed-phase liquid chromatography with porous graphitic carbon liquid chromatography for simultaneous proteomics and N-glycomics analysis.J Chromatogr A. 2015 Oct 9;1415:57-66. doi: 10.1016/j.chroma.2015.08.017. Epub 2015 Aug 19. J Chromatogr A. 2015. PMID: 26362810
-
Zwitterionic hydrophilic interaction liquid chromatography (ZIC-HILIC and ZIC-cHILIC) provide high resolution separation and increase sensitivity in proteome analysis.Anal Chem. 2011 May 1;83(9):3440-7. doi: 10.1021/ac103312e. Epub 2011 Mar 28. Anal Chem. 2011. PMID: 21443167
-
[Stationary phases for hydrophilic interaction liquid chromatography and their applications in separation of traditional Chinese medicines].Se Pu. 2009 Sep;27(5):675-81. Se Pu. 2009. PMID: 20073204 Review. Chinese.
-
Mixed-mode hydrophilic interaction/cation-exchange chromatography (HILIC/CEX) of peptides and proteins.J Sep Sci. 2008 Aug;31(15):2754-73. doi: 10.1002/jssc.200800243. J Sep Sci. 2008. PMID: 18668504 Free PMC article. Review.
Cited by
-
Automated phosphopeptide enrichment from minute quantities of frozen malignant melanoma tissue.PLoS One. 2018 Dec 10;13(12):e0208562. doi: 10.1371/journal.pone.0208562. eCollection 2018. PLoS One. 2018. PMID: 30532160 Free PMC article.
-
What does the future hold for Top Down mass spectrometry?J Am Soc Mass Spectrom. 2010 Feb;21(2):193-202. doi: 10.1016/j.jasms.2009.10.014. Epub 2009 Oct 29. J Am Soc Mass Spectrom. 2010. PMID: 19942451
-
Online coupling of reverse-phase and hydrophilic interaction liquid chromatography for protein and glycoprotein characterization.Anal Bioanal Chem. 2010 Sep;398(2):791-804. doi: 10.1007/s00216-010-3991-2. Epub 2010 Jul 15. Anal Bioanal Chem. 2010. PMID: 20632160 Free PMC article.
-
Integrative network analysis of signaling in human CD34(+) hematopoietic progenitor cells by global phosphoproteomic profiling using TiO2 enrichment combined with 2D LC-MS/MS and pathway mapping.Proteomics. 2013 Apr;13(8):1325-33. doi: 10.1002/pmic.201200369. Epub 2013 Mar 6. Proteomics. 2013. PMID: 23401153 Free PMC article.
-
Quantification of histone modifications using ¹⁵N metabolic labeling.Methods. 2013 Jun 15;61(3):236-43. doi: 10.1016/j.ymeth.2013.02.004. Epub 2013 Feb 27. Methods. 2013. PMID: 23454290 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0021-9673(01)81096-7', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0021-9673(01)81096-7'}]}
- Linden JC, Lawhead CL (1975) J Chromatogr A 105:125–133
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/jssc.200600199', 'is_inner': False, 'url': 'https://doi.org/10.1002/jssc.200600199'}, {'type': 'PubMed', 'value': '16970185', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16970185/'}]}
- Hemström P, Irgum K (2006) J Sep Sci 29:1784–1821 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/ac000338b', 'is_inner': False, 'url': 'https://doi.org/10.1021/ac000338b'}, {'type': 'PubMed', 'value': '11028621', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11028621/'}]}
- Strege MA, Stevenson S, Lawrence SM (2000) Anal Chem 72:4629–4633 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/ac9911490', 'is_inner': False, 'url': 'https://doi.org/10.1021/ac9911490'}, {'type': 'PubMed', 'value': '10784135', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10784135/'}]}
- Risley DS, Strege MA (2000) Anal Chem 72:1736–1739 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.M205166200', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.m205166200'}, {'type': 'PubMed', 'value': '12154089', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12154089/'}]}
- Sarg B, Koutzamani E, Helliger W, Rundquist I, Lindner HH (2002) J Biol Chem 277:39195–39201 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

