Biochemical and aggregation analysis of Bence Jones proteins from different light chain diseases
- PMID: 18266119
- PMCID: PMC2556190
- DOI: 10.1080/13506120701815324
Biochemical and aggregation analysis of Bence Jones proteins from different light chain diseases
Abstract
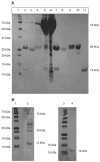
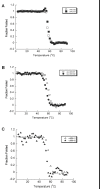
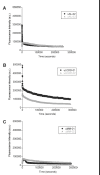
Deposition of immunoglobulin light chains is a result of clonal proliferation of monoclonal plasma cells that secrete free immunoglobulin light chains, also called Bence Jones proteins (BJP). These BJP are present in circulation in large amounts and excreted in urine in various light chain diseases such as light chain amyloidosis (AL), light chain deposition disease (LCDD) and multiple myeloma (MM). BJP from patients with AL, LCDD and MM were purified from their urine and studies were performed to determine their secondary structure, thermodynamic stability and aggregate formation kinetics. Our results show that LCDD and MM proteins have the lowest free energy of folding while all proteins show similar melting temperatures. Incubation of the BJP at their melting temperature produced morphologically different aggregates: amyloid fibrils from the AL proteins, amorphous aggregates from the LCDD proteins and large spherical species from the MM proteins. The aggregates formed under in vitro conditions suggested that the various proteins derived from patients with different light chain diseases might follow different aggregation pathways.
Figures




References
-
- Roussel A, Spinelli S, Deret S, Navaza J, Aucouturier P, Cambillau C. The structure of an entire noncovalent immunoglobulin kappa light-chain dimer (Bence-Jones protein) reveals a weak and unusual constant domains association. Eur J Biochem. 1999;26:192–199. - PubMed
-
- Gertz MA, Lacy MQ, Dispenzieri A, Amyloidosis Hematol Oncol Clin North Am. 1999;13:1211–1233. - PubMed
-
- Sezer O, Eucker J, Schmid P, Possinger K. New therapeutic approaches in primary systemic AL amyloidosis. Ann Hematol. 2000;79:1–6. - PubMed
-
- Roye B, Arnulf B, Martinez F, Roy L, Flageul B, Etienne I, Ronco P, Brouet JC, Fermand JP. High dose chemotherapy in light chain or light and heavy chain deposition disease. Kidney Int. 2004;65:642–648. - PubMed
-
- Wetzel R. Domain stability in immunoglobulin light chain deposition disorders. Adv Protein Chem. 1997;50:183–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
