Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1
- PMID: 18266466
- PMCID: PMC2233671
- DOI: 10.1371/journal.ppat.0040021
Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1
Abstract
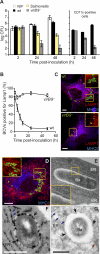
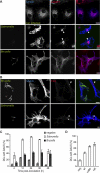
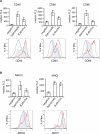
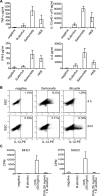
Brucella is an intracellular pathogen able to persist for long periods of time within the host and establish a chronic disease. We show that soon after Brucella inoculation in intestinal loops, dendritic cells from ileal Peyer's patches become infected and constitute a cell target for this pathogen. In vitro, we found that Brucella replicates within dendritic cells and hinders their functional activation. In addition, we identified a new Brucella protein Btp1, which down-modulates maturation of infected dendritic cells by interfering with the TLR2 signaling pathway. These results show that intracellular Brucella is able to control dendritic cell function, which may have important consequences in the development of chronic brucellosis.
Conflict of interest statement
Figures

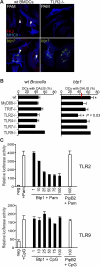
References
-
- Skinner JA, Pilione MR, Shen H, Harvill ET, Yuk MH. Bordetella type III secretion modulates dendritic cell migration resulting in immunosuppression and bacterial persistence. J Immunol. 2005;175:4647–4652. - PubMed
-
- Petrovska L, Aspinall RJ, Barber L, Clare S, Simmons CP, et al. Salmonella enterica serovar Typhimurium interaction with dendritic cells: impact of the sifA gene. Cell Microbiol. 2004;6:1071–1084. - PubMed
-
- Tobar JA, Gonzalez PA, Kalergis AM. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fc gamma receptors on dendritic cells. J Immunol. 2004;173:4058–4065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

