RNA interference-mediated silencing of the polo-like kinase 1 gene enhances chemosensitivity to gemcitabine in pancreatic adenocarcinoma cells
- PMID: 18266952
- PMCID: PMC4514112
- DOI: 10.1111/j.1582-4934.2008.00257.x
RNA interference-mediated silencing of the polo-like kinase 1 gene enhances chemosensitivity to gemcitabine in pancreatic adenocarcinoma cells
Abstract
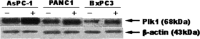
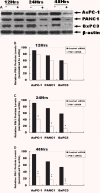
Gemcitabine is the first-line chemotherapeutic agent for advanced adenocarcinoma of the pancreas; however, chemoresistance to gemcitabine remains a major cause of failure for the clinical treatment of this disease. Polo-like kinase 1 (Plk-1) is highly expressed in pancreatic cancer cell lines and pancreatic tumour tissues, and is involved in a wide variety of cell cycle processes. Nevertheless, its biological role and implication for gemcitabine resistance are not clearly defined. In this study, we used RNA-interference (RNAi)-mediated depletion of Plk-1 to determine its potential for sensitizing pancreatic tumour cells to gemcitabine. We showed that the level of Plk-1 protein was correlated significantly with gemcitabine resistance in human pancreatic adenocarcinoma cells and that overexpression of Plk-1 reduced sensitivity to gemcitabine in these cells. In addition, small interfering RNA (siRNA)-mediated knockdown of Plk-1 caused cell cycle arrest at G2/M and the reduction of cellular proliferation. More importantly, the treatment of pancreatic cancer cells with Plk-1 siRNA followed by exposure to gemcitabine dramatically decreased cell viability and increased cellular apoptosis, as compared with treatment with either agent alone. These observations indicate that down-regulation of Plk-1 expression by RNAi enhances gemcitabine sensitivity and increases gemcitabine cytotoxicity in pancreatic tumour cells. This is the first demonstration that the combination of Plk-1 gene therapy and gemcitabine chemotherapy has synergistic anti-tumour activity against pancreatic carcinoma in vitro. This combination treatment warrants further investigation as an effective therapeutic regimen for patients with resistant pancreatic cancer and other tumours.
Figures








Similar articles
-
Inhibition of oncogenic Pim-3 kinase modulates transformed growth and chemosensitizes pancreatic cancer cells to gemcitabine.Cancer Biol Ther. 2013 Jun;14(6):492-501. doi: 10.4161/cbt.24343. Cancer Biol Ther. 2013. PMID: 23760491 Free PMC article.
-
RNA interference demonstrates a novel role for integrin-linked kinase as a determinant of pancreatic adenocarcinoma cell gemcitabine chemoresistance.Clin Cancer Res. 2005 May 1;11(9):3433-8. doi: 10.1158/1078-0432.CCR-04-1510. Clin Cancer Res. 2005. PMID: 15867245 Free PMC article.
-
Validation of Polo-like kinase 1 as a therapeutic target in pancreatic cancer cells.Cancer Biol Ther. 2012 Oct;13(12):1214-20. doi: 10.4161/cbt.21412. Epub 2012 Aug 15. Cancer Biol Ther. 2012. PMID: 22892842 Free PMC article.
-
Updates on treatment of gemcitabine-refractory pancreatic adenocarcinoma. Highlights from the "2011 ASCO Annual Meeting". Chicago, IL, USA; June 3-7, 2011.JOP. 2011 Jul 8;12(4):351-4. JOP. 2011. PMID: 21737894 Review.
-
The Clinical Significance of Phosphorylated Heat Shock Protein 27 (HSPB1) in Pancreatic Cancer.Int J Mol Sci. 2016 Jan 21;17(1):137. doi: 10.3390/ijms17010137. Int J Mol Sci. 2016. PMID: 26805817 Free PMC article. Review.
Cited by
-
Translational Nano-Medicines: Targeted Therapeutic Delivery for Cancer and Inflammatory Diseases.AAPS J. 2015 Jul;17(4):813-27. doi: 10.1208/s12248-015-9772-2. Epub 2015 Apr 29. AAPS J. 2015. PMID: 25921939 Free PMC article. Review.
-
Cross Talk between Wnt/β-Catenin and CIP2A/Plk1 Signaling in Prostate Cancer: Promising Therapeutic Implications.Mol Cell Biol. 2016 May 31;36(12):1734-9. doi: 10.1128/MCB.00130-16. Print 2016 Jun 15. Mol Cell Biol. 2016. PMID: 27090640 Free PMC article. Review.
-
Emodin reverses gemcitabine resistance in pancreatic cancer cells via the mitochondrial apoptosis pathway in vitro.Int J Oncol. 2012 Apr;40(4):1049-57. doi: 10.3892/ijo.2011.1285. Epub 2011 Dec 7. Int J Oncol. 2012. PMID: 22159556 Free PMC article.
-
Regulation of cell apoptosis and proliferation in pancreatic cancer through PI3K/Akt pathway via Polo-like kinase 1.Oncol Rep. 2016 Jul;36(1):49-56. doi: 10.3892/or.2016.4820. Epub 2016 May 18. Oncol Rep. 2016. PMID: 27220401 Free PMC article.
-
The expression of PLK-1 in cervical carcinoma: a possible target for enhancing chemosensitivity.J Exp Clin Cancer Res. 2009 Sep 23;28(1):130. doi: 10.1186/1756-9966-28-130. J Exp Clin Cancer Res. 2009. PMID: 19775446 Free PMC article.
References
-
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. - PubMed
-
- Chu QD, Khushalani N, Javle MM, Douglass HO, Glbbs JF. Should adjuvant therapy remain the standard of care for patients with resected adenocarcinoma of the pancreas? Ann Surg Oncol. 2003;10:539–45. - PubMed
-
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer JCIin. 2003;53:5–26. - PubMed
-
- Kulke MH. Recent developments in the pharmacological treatment of advanced pancreatic cancer. Expert Opin Investig Drugs. 2003;12:983–92. - PubMed
-
- Neoptolemos JP, Cunningham D, Frless H, Bassl C, Stocken DD, Talt DM, Dunn JA, Dervenls C, Lacalne F, Hlckey H, Raraty MG, Ghaneh P, Biichler MW. Adjuvant therapy in pancreatic cancer: historical and current perspectives. Ann Oncol. 2003;14:675–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

