uPA dependent and independent mechanisms of wound healing by C-phycocyanin
- PMID: 18266963
- PMCID: PMC3828884
- DOI: 10.1111/j.1582-4934.2008.00272.x
uPA dependent and independent mechanisms of wound healing by C-phycocyanin
Abstract
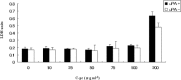
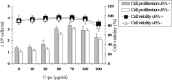
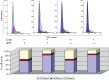
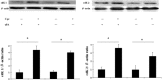
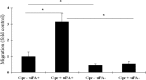
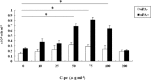
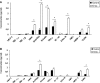
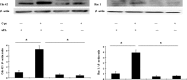
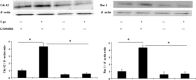
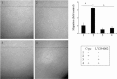
Wound repair requires both recruitment and well co-ordinated actions of many cell types including inflammatory cells, endothelial cells, epithelial cells and importantly fibroblast cells. Urokinase-type plasminogen activator (uPA) system plays a vital role in wound healing phenomenon. We have previously demonstrated that C-phycocyanin (C-pc), a biliprotein from blue-green algae, transcriptionally regulates uPA through cAMP-dependent protein kinase A (PKA) pathway. To date, a role for C-pc in wound-healing scenario is not elucidated. This study was designed to examine the wound-healing property of C-pc in relation to fibroblast proliferation and migration. C-pc increased fibroblast proliferation in a dose-dependent manner. It also enhanced G1 phase of cell cycle and increased the expressions of cyclin-dependent kinases 1 and 2, which facilitate cell cycle progression, in a uPA-independent manner. In vitro wound healing and migration assays revealed the pro-migratory properties of C-pc. Short-interference RNA studies demonstrated that uPA was necessary for C-pc-induced fibroblast migration. C-pc also significantly elevated the expressions of chemokines (MDC, RANTES, Eotaxin, GRO alpha, ENA78 and TARC) and Rho-GTPases (Cdc 42 and Rac 1) in a uPA-dependent manner. Pre-treatment of C-pc-stimulated cells with pharmacological inhibitor of PI-3K (LY294002) annulled the expression of GTPases implying that Rac 1 and Cdc 42 were induced through PI-3K pathway. C-pc-induced cellular migration towards wounded area was also negatively affected by PI-3K inhibition. In vivo wound-healing experiments in mice validated our finding that C-pc accelerates wound healing. Our data provides conclusive evidence of a novel therapeutic usage for C-pc as a wound-healing agent. C-pc is a food and drug administration (FDA)-approved health supplement. We believe this compound can also be beneficial in healing of internal wounds, such as ulcers.
Figures



Similar articles
-
C-phycocyanin transcriptionally regulates uPA mRNA through cAMP mediated PKA pathway in human fibroblast WI-38 cells.Biochim Biophys Acta. 2006 Nov;1760(11):1624-30. doi: 10.1016/j.bbagen.2006.08.012. Epub 2006 Aug 24. Biochim Biophys Acta. 2006. PMID: 17029796
-
The activation of the NF-κB-JNK pathway is independent of the PI3K-Rac1-JNK pathway involved in the bFGF-regulated human fibroblast cell migration.J Dermatol Sci. 2016 Apr;82(1):28-37. doi: 10.1016/j.jdermsci.2016.01.003. Epub 2016 Jan 7. J Dermatol Sci. 2016. PMID: 26829882
-
Downregulation of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 by grape seed proanthocyanidin extract.Phytomedicine. 2010 Jan;17(1):42-6. doi: 10.1016/j.phymed.2009.06.010. Epub 2009 Jul 28. Phytomedicine. 2010. PMID: 19640694
-
Osteopontin: it's role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression.IUBMB Life. 2005 Jun;57(6):441-7. doi: 10.1080/15216540500159424. IUBMB Life. 2005. PMID: 16012053 Review.
-
Primary Ciliary Signaling in the Skin-Contribution to Wound Healing and Scarring.Front Cell Dev Biol. 2020 Nov 13;8:578384. doi: 10.3389/fcell.2020.578384. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33282860 Free PMC article. Review.
Cited by
-
Chitosan Sponges for Efficient Accumulation and Controlled Release of C-Phycocyanin.BioTech (Basel). 2023 Aug 17;12(3):55. doi: 10.3390/biotech12030055. BioTech (Basel). 2023. PMID: 37606442 Free PMC article.
-
Protective Effect of the Phycobiliproteins from Arthrospira maxima on Indomethacin-Induced Gastric Ulcer in a Rat Model.Plants (Basel). 2023 Apr 8;12(8):1586. doi: 10.3390/plants12081586. Plants (Basel). 2023. PMID: 37111811 Free PMC article.
-
Screening Algal and Cyanobacterial Extracts to Identify Potential Substitutes for Fetal Bovine Serum in Cellular Meat Cultivation.Foods. 2024 Nov 22;13(23):3741. doi: 10.3390/foods13233741. Foods. 2024. PMID: 39682813 Free PMC article.
-
MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration.Int Wound J. 2012 Aug;9(4):355-61. doi: 10.1111/j.1742-481X.2011.00890.x. Epub 2011 Nov 9. Int Wound J. 2012. PMID: 22067035 Free PMC article.
-
Nutraceutical Features of the Phycobiliprotein C-Phycocyanin: Evidence from Arthrospira platensis (Spirulina).Nutrients. 2024 Jun 3;16(11):1752. doi: 10.3390/nu16111752. Nutrients. 2024. PMID: 38892686 Free PMC article. Review.
References
-
- Davis GE, Saunders WB. Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Invest Dermatol. 2006;126:44–56. - PubMed
-
- Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31:S213–20. - PubMed
-
- Lamme EN, Van Leeuwen RT, Brandsma K, Van Marle J, Middelkoop E. Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J Pathol. 2000;190:595–603. - PubMed
-
- Nicholl SM, Roztocil E, Davies MG. Urokinase-induced smooth muscle cell responses require distinct signaling pathways: a role for the epidermal growth factor receptor. J Vasc Surg. 2005;41:672–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

