piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning
- PMID: 18267091
- PMCID: PMC2268086
- DOI: 10.1016/j.devcel.2007.11.001
piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning
Abstract
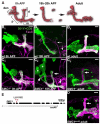
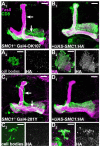
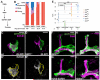
Developmental axon pruning is widely used to refine neural circuits. We performed a mosaic screen to identify mutations affecting axon pruning of Drosophila mushroom body gamma neurons. We constructed a modified piggyBac vector with improved mutagenicity and generated insertions in >2000 genes. We identified two cohesin subunits (SMC1 and SA) as being essential for axon pruning. The cohesin complex maintains sister-chromatid cohesion during cell division in eukaryotes. However, we show that the pruning phenotype in SMC1(-/-) clones is rescued by expressing SMC1 in neurons, revealing a postmitotic function. SMC1(-/-) clones exhibit reduced levels of the ecdysone receptor EcR-B1, a key regulator of axon pruning. The pruning phenotype is significantly suppressed by overexpressing EcR-B1 and is enhanced by a reduced dose of EcR, supporting a causal relationship. We also demonstrate a postmitotic role for SMC1 in dendrite targeting of olfactory projection neurons. We suggest that cohesin regulates diverse aspects of neuronal morphogenesis.
Figures



References
-
- Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. - PubMed
-
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

