The intracellular accumulation of polymeric neuroserpin explains the severity of the dementia FENIB
- PMID: 18267959
- PMCID: PMC2387220
- DOI: 10.1093/hmg/ddn041
The intracellular accumulation of polymeric neuroserpin explains the severity of the dementia FENIB
Abstract
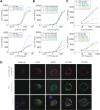
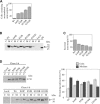
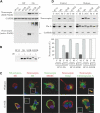
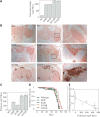
Familial encephalopathy with neuroserpin inclusion bodies (FENIB) is an autosomal dominant dementia that is characterized by the retention of polymers of neuroserpin as inclusions within the endoplasmic reticulum (ER) of neurons. We have developed monoclonal antibodies that detect polymerized neuroserpin and have used COS-7 cells, stably transfected PC12 cell lines and transgenic Drosophila melanogaster to characterize the cellular handling of all four mutant forms of neuroserpin that cause FENIB. We show a direct correlation between the severity of the disease-causing mutation and the accumulation of neuroserpin polymers in cell and fly models of the disease. Moreover, mutant neuroserpin causes locomotor deficits in the fly allowing us to demonstrate a direct link between polymer accumulation and neuronal toxicity.
Figures

References
-
- Carrell R.W., Lomas D.A. Conformational diseases. Lancet. 1997;350:134–138. - PubMed
-
- Kaiserman D., Whisstock J.C., Bird P.I. Mechanisms of serpin dysfunction in disease. Expert Rev. Mol. Med. 2006;8:1–19. - PubMed
-
- Huntington J.A., Pannu N.S., Hazes B., Read R., Lomas D.A., Carrell R.W. A 2.6Å structure of a serpin polymer and implications for conformational disease. J. Mol. Biol. 1999;293:449–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

