Dopamine determines the vulnerability of striatal neurons to the N-terminal fragment of mutant huntingtin through the regulation of mitochondrial complex II
- PMID: 18267960
- PMCID: PMC2367694
- DOI: 10.1093/hmg/ddn033
Dopamine determines the vulnerability of striatal neurons to the N-terminal fragment of mutant huntingtin through the regulation of mitochondrial complex II
Abstract
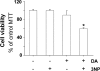
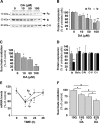
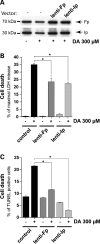
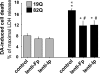
In neurodegenerative disorders associated with primary or secondary mitochondrial defects such as Huntington's disease (HD), cells of the striatum are particularly vulnerable to cell death, although the mechanisms by which this cell death is induced are unclear. Dopamine, found in high concentrations in the striatum, may play a role in striatal cell death. We show that in primary striatal cultures, dopamine increases the toxicity of an N-terminal fragment of mutated huntingtin (Htt-171-82Q). Mitochondrial complex II protein (mCII) levels are reduced in HD striatum, indicating that this protein may be important for dopamine-mediated striatal cell death. We found that dopamine enhances the toxicity of the selective mCII inhibitor, 3-nitropropionic acid. We also demonstrated that dopamine doses that are insufficient to produce cell loss regulate mCII expression at the mRNA, protein and catalytic activity level. We also show that dopamine-induced down-regulation of mCII levels can be blocked by several dopamine D2 receptor antagonists. Sustained overexpression of mCII subunits using lentiviral vectors abrogated the effects of dopamine, both by high dopamine concentrations alone and neuronal death induced by low dopamine concentrations together with Htt-171-82Q. This novel pathway links dopamine signaling and regulation of mCII activity and could play a key role in oxidative energy metabolism and explain the vulnerability of the striatum in neurodegenerative diseases.
Figures



References
-
- Beal M.F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann. Neurol. 1992;31:119–130. - PubMed
-
- Harper P.S. Huntington's Disease. London: WB Saunders Company Ltd; 1991.
-
- Ferrante R.J., Kowall N.W., Beal M.F., Martin J.B., Bird E.D., Richardson E.P., Jr Morphologic and histochemical characteristics of a spared subset of striatal neurons in Huntington's disease. J. Neuropathol. Exp. Neurol. 1987;46:12–27. - PubMed
-
- The Huntington Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. - PubMed
-
- Graham R.K., Deng Y., Slow E.J., Haigh B., Bissada N., Lu G., Pearson J., Shehadeh J., Bertram L., Murphy Z., et al. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell. 2006;125:1179–1191. - PubMed

