Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence
- PMID: 18267966
- PMCID: PMC2330248
- DOI: 10.1093/nar/gkn050
Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence
Abstract
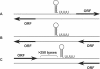
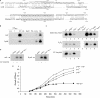
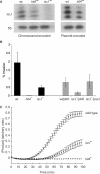
The emergence of pathogenic strains of enteric bacteria and their adaptation to unique niches are associated with the acquisition of foreign DNA segments termed 'genetic islands'. We explored these islands for the occurrence of small RNA (sRNA) encoding genes. Previous systematic screens for enteric bacteria sRNAs were mainly carried out using the laboratory strain Escherichia coli K12, leading to the discovery of approximately 80 new sRNA genes. These searches were based on conservation within closely related members of enteric bacteria and thus, sRNAs, unique to pathogenic strains were excluded. Here we describe the identification and characterization of 19 novel unique sRNA genes encoded within the 'genetic islands' of the virulent strain Salmonella typhimurium. We show that the expression of many of the island-encoded genes is associated with stress conditions and stationary phase. Several of these sRNA genes are induced when Salmonella resides within macrophages. One sRNA, IsrJ, was further examined and found to affect the translocation efficiency of virulence-associated effector proteins into nonphagocytic cells. In addition, we report that unlike the majority of the E. coli sRNAs that are trans regulators, many of the island-encoded sRNAs affect the expression of cis-encoded genes. Our study suggests that the island encoded sRNA genes play an important role within the network that regulates bacterial adaptation to environmental changes and stress conditions and thus controls virulence.
Figures


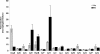
Similar articles
-
A Salmonella small non-coding RNA facilitates bacterial invasion and intracellular replication by modulating the expression of virulence factors.PLoS Pathog. 2011 Sep;7(9):e1002120. doi: 10.1371/journal.ppat.1002120. Epub 2011 Sep 15. PLoS Pathog. 2011. PMID: 21949647 Free PMC article.
-
6S RNA is involved in acid resistance and invasion of epithelial cells in Salmonella enterica serovar Typhimurium.Future Microbiol. 2017 Sep;12:1045-1057. doi: 10.2217/fmb-2017-0055. Epub 2017 Aug 10. Future Microbiol. 2017. PMID: 28796533
-
Dynamics of Salmonella small RNA expression in non-growing bacteria located inside eukaryotic cells.RNA Biol. 2012 Apr;9(4):469-88. doi: 10.4161/rna.19317. Epub 2012 Feb 16. RNA Biol. 2012. PMID: 22336761
-
Identification of bacterial small non-coding RNAs: experimental approaches.Curr Opin Microbiol. 2007 Jun;10(3):257-61. doi: 10.1016/j.mib.2007.05.003. Epub 2007 Jun 5. Curr Opin Microbiol. 2007. PMID: 17553733 Review.
-
The expanding targetome of small RNAs in Salmonella Typhimurium.Biochimie. 2017 Jun;137:69-77. doi: 10.1016/j.biochi.2017.03.005. Epub 2017 Mar 14. Biochimie. 2017. PMID: 28302471 Review.
Cited by
-
RNA-based mechanisms of virulence control in Enterobacteriaceae.RNA Biol. 2017 May 4;14(5):471-487. doi: 10.1080/15476286.2016.1201617. Epub 2016 Jul 21. RNA Biol. 2017. PMID: 27442607 Free PMC article. Review.
-
The Small RNA RyhB Homologs from Salmonella Typhimurium Restrain the Intracellular Growth and Modulate the SPI-1 Gene Expression within RAW264.7 Macrophages.Microorganisms. 2021 Mar 18;9(3):635. doi: 10.3390/microorganisms9030635. Microorganisms. 2021. PMID: 33803635 Free PMC article.
-
Characterisation of sRNAs enriched in outer membrane vesicles of pathogenic Flavobacterium psychrophilum causing Bacterial Cold Water Disease in rainbow trout.J Extracell Biol. 2024 Jun 19;3(6):e161. doi: 10.1002/jex2.161. eCollection 2024 Jun. J Extracell Biol. 2024. PMID: 38947174 Free PMC article.
-
An integrated approach for finding overlooked genes in Shigella.PLoS One. 2011 Apr 5;6(4):e18509. doi: 10.1371/journal.pone.0018509. PLoS One. 2011. PMID: 21483688 Free PMC article.
-
The Role of Ribonucleases and sRNAs in the Virulence of Foodborne Pathogens.Front Microbiol. 2017 May 19;8:910. doi: 10.3389/fmicb.2017.00910. eCollection 2017. Front Microbiol. 2017. PMID: 28579982 Free PMC article. Review.
References
-
- Gerdes K, Wagner EG. RNA antitoxins. Curr. Opin. Microbiol. 2007;10:117–124. - PubMed
-
- Masse E, Salvail H, Desnoyers G, Arguin M. Small RNAs controlling iron metabolism. Curr. Opin. Microbiol. 2007;10:140–145. - PubMed
-
- Valentin-Hansen P, Johansen J, Rasmussen AA. Small RNAs controlling outer membrane porins. Curr. Opin. Microbiol. 2007;10:152–155. - PubMed
-
- Vanderpool CK. Physiological consequences of small RNA-mediated regulation of glucose-phosphate stress. Curr. Opin. Microbiol. 2007;10:146–151. - PubMed
-
- Vogel J, Papenfort K. Small non-coding RNAs and the bacterial outer membrane. Curr. Opin. Microbiol. 2006;9:605–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

