Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity
- PMID: 18268005
- PMCID: PMC2293116
- DOI: 10.1128/MCB.01601-07
Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity
Abstract
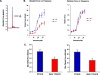
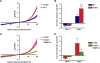
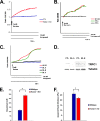
Transient receptor potential (TRP) channels are nonselective cation channels, several of which are expressed in striated muscle. Because the scaffolding protein Homer 1 has been implicated in TRP channel regulation, we hypothesized that Homer proteins play a significant role in skeletal muscle function. Mice lacking Homer 1 exhibited a myopathy characterized by decreased muscle fiber cross-sectional area and decreased skeletal muscle force generation. Homer 1 knockout myotubes displayed increased basal current density and spontaneous cation influx. This spontaneous cation influx in Homer 1 knockout myotubes was blocked by reexpression of Homer 1b, but not Homer 1a, and by gene silencing of TRPC1. Moreover, diminished Homer 1 expression in mouse models of Duchenne's muscular dystrophy suggests that loss of Homer 1 scaffolding of TRP channels may contribute to the increased stretch-activated channel activity observed in mdx myofibers. These findings provide direct evidence that Homer 1 functions as an important scaffold for TRP channels and regulates mechanotransduction in skeletal muscle.
Figures




References
-
- Ango, F., L. Prezeau, T. Muller, J. C. Tu, B. Xiao, P. F. Worley, J. P. Pin, J. Bockaert, and L. Fagni. 2001. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411962-965. - PubMed
-
- Berthier, C., and S. Blaineau. 1997. Supramolecular organization of the subsarcolemmal cytoskeleton of adult skeletal muscle fibers. A review. Biol. Cell 89413-434. - PubMed
-
- Bloch, R. J., P. Reed, A. O'Neill, J. Strong, M. Williams, N. Porter, and H. Gonzalez-Serratos. 2004. Costameres mediate force transduction in healthy skeletal muscle and are altered in muscular dystrophies. J. Muscle Res. Cell Motil. 25590-592. - PubMed
-
- Bortoloso, E., N. Pilati, A. Megighian, E. Tibaldo, D. Sandona, and P. Volpe. 2006. Transition of Homer isoforms during skeletal muscle regeneration. Am. J. Physiol. 290C711—C718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
