Activated Kras, but not Hras or Nras, may initiate tumors of endodermal origin via stem cell expansion
- PMID: 18268007
- PMCID: PMC2293097
- DOI: 10.1128/MCB.01661-07
Activated Kras, but not Hras or Nras, may initiate tumors of endodermal origin via stem cell expansion
Abstract
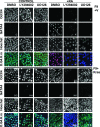
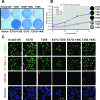
The three closely related human Ras genes, Hras, Nras, and Kras, are all widely expressed, engage a common set of downstream effectors, and can each exhibit oncogenic activity. However, the vast majority of activating Ras mutations in human tumors involve Kras. Moreover, Kras mutations are most frequently seen in tumors of endodermally derived tissues (lung, pancreas, and colon), suggesting that activated Kras may affect an endodermal progenitor to initiate oncogenesis. Using a culture model of retinoic acid (RA)-induced stem cell differentiation to endoderm, we determined that while activated HrasV12 promotes differentiation and growth arrest in these endodermal progenitors, KrasV12 promotes their proliferation. Furthermore, KrasV12-expressing endodermal progenitors fail to differentiate upon RA treatment and continue to proliferate and maintain stem cell characteristics. NrasV12 neither promotes nor prevents differentiation. A structure-function analysis demonstrated that these distinct effects of the Ras isoforms involve their variable C-terminal domains, implicating compartmentalized signaling, and revealed a requirement for several established Ras effectors. These findings indicate that activated Ras isoforms exert profoundly different effects on endodermal progenitors and that mutant Kras may initiate tumorigenesis by expanding a susceptible stem/progenitor cell population. These results potentially explain the high frequency of Kras mutations in tumors of endodermal origin.
Figures
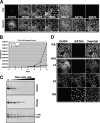
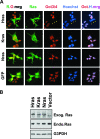
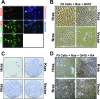



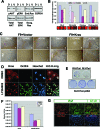

References
-
- Adjei, A. A. 2001. Blocking oncogenic Ras signaling for cancer therapy. J. Natl. Cancer Inst. 931062-1074. - PubMed
-
- Adjei, A. A. 2001. Ras signaling pathway proteins as therapeutic targets. Curr. Pharm. Des. 71581-1594. - PubMed
-
- Agbunag, C., and D. Bar-Sagi. 2004. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 645659-5663. - PubMed
-
- Bar-Sagi, D., and J. R. Feramisco. 1985. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell 42841-848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
