Rgs5 targeting leads to chronic low blood pressure and a lean body habitus
- PMID: 18268011
- PMCID: PMC2293121
- DOI: 10.1128/MCB.01889-07
Rgs5 targeting leads to chronic low blood pressure and a lean body habitus
Abstract
RGS5 is a potent GTPase-activating protein for G(ialpha) and G(qalpha) that is expressed strongly in pericytes and is present in vascular smooth muscle cells. To study the role of RGS5 in blood vessel physiology, we generated Rgs5-deficient mice. The Rgs5(-/-) mice developed normally, without obvious defects in cardiovascular development or function. Surprisingly, Rgs5(-/-) mice had persistently low blood pressure, lower in female mice than in male mice, without concomitant cardiac dysfunction, and a lean body habitus. The examination of the major blood vessels revealed that the aortas of Rgs5(-/-) mice were dilated compared to those of control mice, without altered wall thickness. Isolated aortic smooth muscle cells from the Rgs5(-/-) mice exhibited exaggerated levels of phosphorylation of vasodilator-stimulated phosphoprotein and extracellular signal-regulated kinase in response to stimulation with either sodium nitroprusside or sphingosine 1-phosphate. The results of this study, along with those of previous studies demonstrating that RGS5 stability is under the control of nitric oxide via the N-end rule pathway, suggest that RGS5 may balance vascular tone by attenuating vasodilatory signaling in vivo in opposition to RGS2, another RGS (regulator of G protein signaling) family member known to inhibit G protein-coupled receptor-mediated vasoconstrictor signaling. Blocking the function or the expression of RGS5 may provide an alternative approach to treat hypertension.
Figures
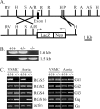
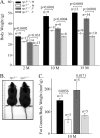
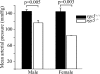
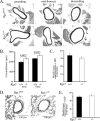

References
-
- Adams, L. D., R. L. Geary, B. McManus, and S. M. Schwartz. 2000. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ. Res. 87623-631. - PubMed
-
- Adams, L. D., R. L. Geary, J. Li, A. Rossini, and S. M. Schwartz. 2006. Expression profiling identifies smooth muscle cell diversity within human intima and plaque fibrous cap: loss of RGS5 distinguishes the cap. Arterioscler. Thromb. Vasc. Biol. 26319-325. - PubMed
-
- Bassil, M., and M. B. Anand-Srivastava. 2006. Nitric oxide modulates Gi-protein expression and adenylyl cyclase signaling in vascular smooth muscle cells. Free Radic. Biol. Med. 411162-1173. - PubMed
-
- Berger, M., G. Bergers, B. Arnold, G. J. Hammerling, and R. Ganss. 2005. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood 1051094-1101. - PubMed
-
- Bondjers, C., M. Kalen, M. Hellstrom, S. J. Scheidl, A. Abramsson, O. Renner, P. Lindahl, H. Cho, J. Kehrl, and C. Betsholtz. 2003. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am. J. Pathol. 162721-729. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
