Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal
- PMID: 18268013
- PMCID: PMC2293104
- DOI: 10.1128/MCB.01795-07
Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal
Abstract
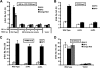
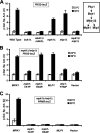
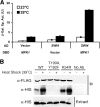
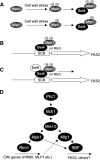
The cell wall integrity mitogen-activated protein kinase (MAPK) cascade of Saccharomyces cerevisiae drives changes in gene expression in response to cell wall stress. We show that the MAPK of this pathway (Mpk1) and its pseudokinase paralog (Mlp1) use a noncatalytic mechanism to activate transcription of the FKS2 gene. Transcriptional activation of FKS2 was dependent on the Swi4/Swi6 (SBF) transcription factor and on an activating signal to Mpk1 but not on protein kinase activity. Activated (phosphorylated) Mpk1 and Mlp1 were detected in a complex with Swi4 and Swi6 at the FKS2 promoter. Mpk1 association with Swi4 in vivo required phosphorylation of Mpk1. Promoter association of Mpk1 and the Swi4 DNA-binding subunit of SBF were codependent but did not require Swi6, indicating that the MAPK confers DNA-binding ability to Swi4. Based on these data, we propose a model in which phosphorylated Mpk1 or Mlp1 forms a dimeric complex with Swi4 that is competent to associate with the FKS2 promoter. This complex then recruits Swi6 to activate transcription. Finally, we show that human ERK5, a functional ortholog of Mpk1, is similarly capable of driving FKS2 expression in the absence of protein kinase activity, suggesting that this mammalian MAPK may also have a noncatalytic function in vivo.
Figures


Similar articles
-
Yeast Mpk1 cell wall integrity mitogen-activated protein kinase regulates nucleocytoplasmic shuttling of the Swi6 transcriptional regulator.Mol Biol Cell. 2010 May 1;21(9):1609-19. doi: 10.1091/mbc.e09-11-0923. Epub 2010 Mar 10. Mol Biol Cell. 2010. PMID: 20219973 Free PMC article.
-
Mechanism of Mpk1 mitogen-activated protein kinase binding to the Swi4 transcription factor and its regulation by a novel caffeine-induced phosphorylation.Mol Cell Biol. 2009 Dec;29(24):6449-61. doi: 10.1128/MCB.00794-09. Epub 2009 Oct 5. Mol Cell Biol. 2009. PMID: 19805511 Free PMC article.
-
Transcriptional reporters for genes activated by cell wall stress through a non-catalytic mechanism involving Mpk1 and SBF.Yeast. 2010 Aug;27(8):541-8. doi: 10.1002/yea.1782. Yeast. 2010. PMID: 20641022 Free PMC article.
-
Control of Gene Expression via the Yeast CWI Pathway.Int J Mol Sci. 2022 Feb 4;23(3):1791. doi: 10.3390/ijms23031791. Int J Mol Sci. 2022. PMID: 35163713 Free PMC article. Review.
-
A walk-through MAPK structure and functionality with the 30-year-old yeast MAPK Slt2.Int Microbiol. 2021 Nov;24(4):531-543. doi: 10.1007/s10123-021-00183-z. Epub 2021 May 15. Int Microbiol. 2021. PMID: 33993419 Review.
Cited by
-
Chromatin remodeling by the SWI/SNF complex is essential for transcription mediated by the yeast cell wall integrity MAPK pathway.Mol Biol Cell. 2012 Jul;23(14):2805-17. doi: 10.1091/mbc.E12-04-0278. Epub 2012 May 23. Mol Biol Cell. 2012. PMID: 22621902 Free PMC article.
-
Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway.Genetics. 2011 Dec;189(4):1145-75. doi: 10.1534/genetics.111.128264. Genetics. 2011. PMID: 22174182 Free PMC article. Review.
-
Variants of the yeast MAPK Mpk1 are fully functional independently of activation loop phosphorylation.Mol Biol Cell. 2016 Sep 1;27(17):2771-83. doi: 10.1091/mbc.E16-03-0167. Epub 2016 Jul 13. Mol Biol Cell. 2016. PMID: 27413009 Free PMC article.
-
Signal inhibition by a dynamically regulated pool of monophosphorylated MAPK.Mol Biol Cell. 2015 Sep 15;26(18):3359-71. doi: 10.1091/mbc.E15-01-0037. Epub 2015 Jul 15. Mol Biol Cell. 2015. PMID: 26179917 Free PMC article.
-
The Farnesyltransferase β-Subunit Ram1 Regulates Sporisorium scitamineum Mating, Pathogenicity and Cell Wall Integrity.Front Microbiol. 2019 May 8;10:976. doi: 10.3389/fmicb.2019.00976. eCollection 2019. Front Microbiol. 2019. PMID: 31134021 Free PMC article.
References
-
- Abe, J., M. Kusuhara, R. J. Ulevitch, B. C. Berk, and J. D. Lee. 1996. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem. 27116586-16590. - PubMed
-
- Alepuz, P. M., A. Jovanovic, V. Reiser, and G. Ammerer. 2001. Stress-induced MAP kinase Hog1 is part of transcription activation complexes. Mol. Cell 7767-777. - PubMed
-
- Andrews, B. J., and L. A. Moore. 1992b. Mutational analysis of a DNA sequence involved in linking gene expression to the cell cycle. Biochem. Cell Biol. 701073-1080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
