VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma
- PMID: 18268040
- PMCID: PMC2271023
- DOI: 10.1084/jem.20072041
VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma
Abstract
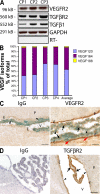
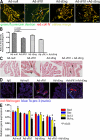
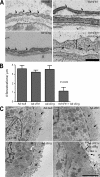
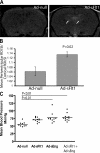
Although the role of vascular endothelial growth factor (VEGF) in developmental and pathological angiogenesis is well established, its function in the adult is less clear. Similarly, although transforming growth factor (TGF) beta is involved in angiogenesis, presumably by mediating capillary (endothelial cell [EC]) stability, its involvement in quiescent vasculature is virtually uninvestigated. Given the neurological findings in patients treated with VEGF-neutralizing therapy (bevacizumab) and in patients with severe preeclampsia, which is mediated by soluble VEGF receptor 1/soluble Fms-like tyrosine kinase receptor 1 and soluble endoglin, a TGF-beta signaling inhibitor, we investigated the roles of VEGF and TGF-beta in choroid plexus (CP) integrity and function in adult mice. Receptors for VEGF and TGF-beta were detected in adult CP, as well as on ependymal cells. Inhibition of VEGF led to decreased CP vascular perfusion, which was associated with fibrin deposition. Simultaneous blockade of VEGF and TGF-beta resulted in the loss of fenestrae on CP vasculature and thickening of the otherwise attenuated capillary endothelium, as well as the disappearance of ependymal cell microvilli and the development of periventricular edema. These results provide compelling evidence that both VEGF and TGF-beta are involved in the regulation of EC stability, ependymal cell function, and periventricular permeability.
Figures

References
-
- Coultas, L., K. Chawengsaksophak, and J. Rossant. 2005. Endothelial cells and VEGF in vascular development. Nature. 438:937–945. - PubMed
-
- Carmeliet, P., V. Ferreira, G. Breier, S. Pollefeyt, L. Kieckens, M. Gertsenstein, M. Fahrig, A. Vandenhoeck, K. Harpal, C. Eberhardt, et al. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380:435–439. - PubMed
-
- Ferrara, N., K. Carver-Moore, H. Chen, M. Dowd, L. Lu, K.S. O'Shea, L. Powell-Braxton, K.J. Hillan, and M.W. Moore. 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 380:439–442. - PubMed
-
- Miquerol, L., B.L. Langille, and A. Nagy. 2000. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 127:3941–3946. - PubMed
-
- Eming, S.A., and T. Krieg. 2006. Molecular mechanisms of VEGF-A action during tissue repair. J. Investig. Dermatol. Symp. Proc. 11:79–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

