Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials
- PMID: 18268087
- PMCID: PMC2292547
- DOI: 10.1128/AAC.01033-07
Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials
Abstract
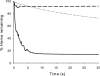
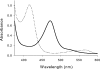
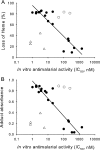
The reaction of spiro- and dispiro-1,2,4-trioxolane antimalarials with heme has been investigated to provide further insight into the mechanism of action for this important class of antimalarials. A series of trioxolanes with various antimalarial potencies was found to be unreactive in the presence of Fe(III) hemin, but all were rapidly degraded by reduced Fe(II) heme. The major reaction product from the heme-mediated degradation of biologically active trioxolanes was an alkylated heme adduct resulting from addition of a radical intermediate. Under standardized reaction conditions, a correlation (R2 = 0.88) was found between the extent of heme alkylation and in vitro antimalarial activity, suggesting that heme alkylation may be related to the mechanism of action for these trioxolanes. Significantly less heme alkylation was observed for the clinically utilized artemisinin derivatives compared to the equipotent trioxolanes included in this study.
Figures

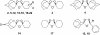
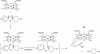
References
-
- Atamna, H., and H. Ginsburg. 1995. Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J. Biol. Chem. 270:24876-24883. - PubMed
-
- Campanale, N., C. Nickel, C. A. Daubenberger, D. A. Wehlan, J. J. Gorman, N. Klonis, K. Becker, and L. Tilley. 2003. Identification and characterization of heme-interacting proteins in the malaria parasite, Plasmodium falciparum. J. Biol. Chem. 278:27354-27361. - PubMed
-
- Creek, D. J., W. N. Charman, F. C. K. Chiu, R. J. Prankerd, K. J. McCullough, Y. Dong, J. L. Vennerstrom, and S. A. Charman. 2007. Iron-mediated degradation kinetics of substituted dispiro-1,2,4-trioxolane antimalarials. J. Pharm. Sci. 96:2945-2956. - PubMed
-
- Creek, D. J., F. C. K. Chiu, R. J. Prankerd, S. A. Charman, and W. N. Charman. 2005. Kinetics of iron-mediated artemisinin degradation: effect of solvent composition and iron salt. J. Pharm. Sci. 94:1820-1829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

