Akt regulates centrosome migration and spindle orientation in the early Drosophila melanogaster embryo
- PMID: 18268102
- PMCID: PMC2234228
- DOI: 10.1083/jcb.200705085
Akt regulates centrosome migration and spindle orientation in the early Drosophila melanogaster embryo
Abstract
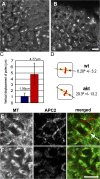
Correct positioning and morphology of the mitotic spindle is achieved through regulating the interaction between microtubules (MTs) and cortical actin. Here we find that, in the Drosophila melanogaster early embryo, reduced levels of the protein kinase Akt result in incomplete centrosome migration around cortical nuclei, bent mitotic spindles, and loss of nuclei into the interior of the embryo. We show that Akt is enriched at the embryonic cortex and is required for phosphorylation of the glycogen synthase kinase-3beta homologue Zeste-white 3 kinase (Zw3) and for the cortical localizations of the adenomatosis polyposis coli (APC)-related protein APC2/E-APC and the MT + Tip protein EB1. We also show that reduced levels of Akt result in mislocalization of APC2 in postcellularized embryonic mitoses and misorientation of epithelial mitotic spindles. Together, our results suggest that Akt regulates a complex containing Zw3, Armadillo, APC2, and EB1 and that this complex has a role in stabilizing MT-cortex interactions, facilitating both centrosome separation and mitotic spindle orientation.
Figures









References
-
- Allan, V., and I.S. Nathke. 2001. Catch and pull a microtubule: getting a grasp on the cortex. Nat. Cell Biol. 3:E226–E228. - PubMed
-
- Andjelkovic, M., D.R. Alessi, R. Meier, A. Fernandez, N.J. Lamb, M. Frech, P. Cron, P. Cohen, J.M. Lucocq, and B.A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515–31524. - PubMed
-
- Berrueta, L., J.S. Tirnauer, S.C. Schuyler, D. Pellman, and B.E. Bierer. 1999. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr. Biol. 9:425–428. - PubMed
-
- Brunet, A., A. Bonni, M.J. Zigmond, M.Z. Lin, P. Juo, L.S. Hu, M.J. Anderson, K.C. Arden, J. Blenis, and M.E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 96:857–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

