Microtubule stabilization specifies initial neuronal polarization
- PMID: 18268107
- PMCID: PMC2234250
- DOI: 10.1083/jcb.200707042
Microtubule stabilization specifies initial neuronal polarization
Abstract
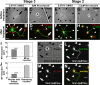
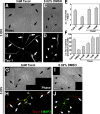
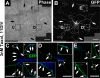
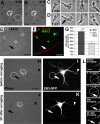
Axon formation is the initial step in establishing neuronal polarity. We examine here the role of microtubule dynamics in neuronal polarization using hippocampal neurons in culture. We see increased microtubule stability along the shaft in a single neurite before axon formation and in the axon of morphologically polarized cells. Loss of polarity or formation of multiple axons after manipulation of neuronal polarity regulators, synapses of amphids defective (SAD) kinases, and glycogen synthase kinase-3beta correlates with characteristic changes in microtubule turnover. Consistently, changing the microtubule dynamics is sufficient to alter neuronal polarization. Application of low doses of the microtubule-destabilizing drug nocodazole selectively reduces the formation of future dendrites. Conversely, low doses of the microtubule-stabilizing drug taxol shift polymerizing microtubules from neurite shafts to process tips and lead to the formation of multiple axons. Finally, local stabilization of microtubules using a photoactivatable analogue of taxol induces axon formation from the activated area. Thus, local microtubule stabilization in one neurite is a physiological signal specifying neuronal polarization.
Figures




References
-
- Arimura, N., and K. Kaibuchi. 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8:194–205. - PubMed
-
- Arregui, C., J. Busciglio, A. Caceres, and H.S. Barra. 1991. Tyrosinated and detyrosinated microtubules in axonal processes of cerebellar macroneurons grown in culture. J. Neurosci. Res. 28:171–181. - PubMed
-
- Basu, R., and F. Chang. 2007. Shaping the actin cytoskeleton using microtubule tips. Curr. Opin. Cell Biol. 19:88–94. - PubMed
-
- Bito, H., T. Furuyashiki, H. Ishihara, Y. Shibasaki, K. Ohashi, K. Mizuno, M. Maekawa, T. Ishizaki, and S. Narumiya. 2000. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 26:431–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

