Optimizing the use of prescription drugs in Canada through the Common Drug Review
- PMID: 18268271
- PMCID: PMC2228339
- DOI: 10.1503/cmaj.070713
Optimizing the use of prescription drugs in Canada through the Common Drug Review
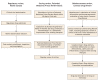
Figures
References
-
- Canadian Institute for Health Information. Drug expenditure in Canada, 1985 to 2006. Ottawa: The Institute; 2007.
-
- Donaldson C, Shackley P. Economic evaluation. In: Detels R, Holland WW, McEwan J, et al, editors. Oxford textbook of public health. 3rd ed. Oxford: Oxford University Press; 1997. p. 849-70.
-
- Canadian Agency for Drugs and Technologies in Health. CADTH presentation on the Common Drug Review to the House of Commons Standing Committee on Health. Ottawa: The Agency; 2007. Available: www.cadth.ca/media/media/CADTH_Submission_to_Standing_Committee_on_Healt... (accessed 2007 Nov 22).
-
- Kallah J. Formulary acceptance: monitoring and evaluation. Provincial Reimbursement Advisor 2006 Nov 9(4):57-77.
-
- Morgan SG, McMahon M, Mitton C, et al. Centralized drug review processes in Australia, Canada, New Zealand, and the United Kingdom. Health Aff 2006;25:337-47. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources