Remodeling of ryanodine receptor complex causes "leaky" channels: a molecular mechanism for decreased exercise capacity
- PMID: 18268335
- PMCID: PMC2538898
- DOI: 10.1073/pnas.0711074105
Remodeling of ryanodine receptor complex causes "leaky" channels: a molecular mechanism for decreased exercise capacity
Abstract
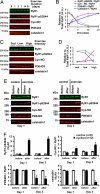
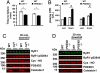
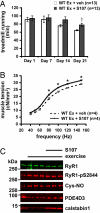
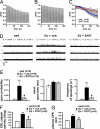
During exercise, defects in calcium (Ca2+) release have been proposed to impair muscle function. Here, we show that during exercise in mice and humans, the major Ca2+ release channel required for excitation-contraction coupling (ECC) in skeletal muscle, the ryanodine receptor (RyR1), is progressively PKA-hyperphosphorylated, S-nitrosylated, and depleted of the phosphodiesterase PDE4D3 and the RyR1 stabilizing subunit calstabin1 (FKBP12), resulting in "leaky" channels that cause decreased exercise tolerance in mice. Mice with skeletal muscle-specific calstabin1 deletion or PDE4D deficiency exhibited significantly impaired exercise capacity. A small molecule (S107) that prevents depletion of calstabin1 from the RyR1 complex improved force generation and exercise capacity, reduced Ca2+-dependent neutral protease calpain activity and plasma creatine kinase levels. Taken together, these data suggest a possible mechanism by which Ca2+ leak via calstabin1-depleted RyR1 channels leads to defective Ca2+ signaling, muscle damage, and impaired exercise capacity.
Conflict of interest statement
Conflict of interest statement: A.R.M. and D.W.L. are on the scientific advisory board and own shares in ARMGO Pharma, Inc., a start-up company that is developing RyR targeted drugs for clinical use in the treatment of heart failure and sudden death. S.R. is a consultant for ARMGO Pharma, Inc.
Figures
References
-
- Brillantes AB, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. - PubMed
-
- Marx SO, Ondrias K, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science. 1998;281:818–821. - PubMed
-
- Avila G, Lee EH, Perez CF, Allen PD, Dirksen RT. FKBP12 binding to RyR1 modulates excitation–contraction coupling in mouse skeletal myotubes. J Biol Chem. 2003;278:22600–22608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

