Hydrophobicity of protein surfaces: Separating geometry from chemistry
- PMID: 18268339
- PMCID: PMC2268126
- DOI: 10.1073/pnas.0708088105
Hydrophobicity of protein surfaces: Separating geometry from chemistry
Abstract
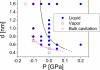
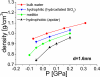
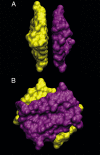
To better understand the role of surface chemical heterogeneity in natural nanoscale hydration, we study via molecular dynamics simulation the structure and thermodynamics of water confined between two protein-like surfaces. Each surface is constructed to have interactions with water corresponding to those of the putative hydrophobic surface of a melittin dimer, but is flattened rather than having its native "cupped" configuration. Furthermore, peripheral charged groups are removed. Thus, the role of a rough surface topography is removed, and results can be productively compared with those previously observed for idealized, atomically smooth hydrophilic and hydrophobic flat surfaces. The results indicate that the protein surface is less hydrophobic than the idealized counterpart. The density and compressibility of water adjacent to a melittin dimer is intermediate between that observed adjacent to idealized hydrophobic or hydrophilic surfaces. We find that solvent evacuation of the hydrophobic gap (cavitation) between dimers is observed when the gap has closed to sterically permit a single water layer. This cavitation occurs at smaller pressures and separations than in the case of idealized hydrophobic flat surfaces. The vapor phase between the melittin dimers occupies a much smaller lateral region than in the case of the idealized surfaces; cavitation is localized in a narrow central region between the dimers, where an apolar amino acid is located. When that amino acid is replaced by a polar residue, cavitation is no longer observed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
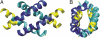


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

