A dose-ranging study of tiotropium delivered via Respimat Soft Mist Inhaler or HandiHaler in COPD patients
- PMID: 18268929
- PMCID: PMC2699972
A dose-ranging study of tiotropium delivered via Respimat Soft Mist Inhaler or HandiHaler in COPD patients
Abstract
This was a multicenter, randomized, double-blind within device, parallel-group, dose-ranging study. COPD patients (n = 202; 86% male; mean age: 61 years) were randomized to receive tiotropium 1.25 microg, 2.5 microg, 5 microg, 10 microg, or 20 microg Respimat SMI (a novel, propellant-free device); tiotropium 18 microg HandiHaler; placebo Respimat; or placebo HandiHaler for 3 weeks. The primary endpoint was trough FEV1 on Day 21. Other assessments included FVC, PEFR, rescue medication use, safety, and pharmacokinetics. In general, all active treatments improved the primary and secondary endpoints on Day 21 (steady state) compared with placebo. Tiotropium 5 microg Respimat, 20 microg Respimat, and tiotropium 18 microg HandiHaler were statistically significantly higher than placebo for the primary endpoint (mean change in trough FEV1 was 150 mL (both Respimat doses) versus 20 mL (placebo Respimat); p < 0.05; and 230 mL (HandiHaler) versus -90 mL (placebo HandiHaler); p < or = 0.001). The urinary excretion (up to 2 hours post-dose) of tiotropium 5-10 microg Respimat was comparable with tiotropium 18 microg HandiHaler; the overall incidence of adverse events was comparable across treatment groups. Tiotropium 5 and 10 microg Respimat improve lung function in COPD patients and appear to be comparable with tiotropium 18 microg HandiHaler.
Figures

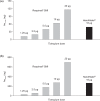
References
-
- [ATS] American Thoracic Society Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. - PubMed
-
- Bretz F, Schmidli H, Konig F, et al. Confirmatory seamless phase II/III clinical trials with hypotheses selection at interim: general concepts. Biom J. 2006;48:623–34. - PubMed
-
- Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–24. - PubMed
-
- Dalby R, Spallek M, Voshaar T. A review of the development of Respimat® Soft MistTM Inhaler. Int J Pharm. 2004;283:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

