Atlastin GTPases are required for Golgi apparatus and ER morphogenesis
- PMID: 18270207
- PMCID: PMC2902292
- DOI: 10.1093/hmg/ddn046
Atlastin GTPases are required for Golgi apparatus and ER morphogenesis
Abstract
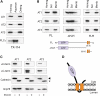
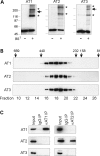
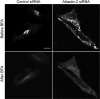
The hereditary spastic paraplegias (SPG1-33) comprise a cluster of inherited neurological disorders characterized principally by lower extremity spasticity and weakness due to a length-dependent, retrograde axonopathy of corticospinal motor neurons. Mutations in the gene encoding the large oligomeric GTPase atlastin-1 are responsible for SPG3A, a common autosomal dominant hereditary spastic paraplegia. Here we describe a family of human GTPases, atlastin-2 and -3 that are closely related to atlastin-1. Interestingly, while atlastin-1 is predominantly localized to vesicular tubular complexes and cis-Golgi cisternae, mostly in brain, atlastin-2 and -3 are localized to the endoplasmic reticulum (ER) and are most enriched in other tissues. Knockdown of atlastin-2 and -3 levels in HeLa cells using siRNA (small interfering RNA) causes disruption of Golgi morphology, and these Golgi structures remain sensitive to brefeldin A treatment. Interestingly, expression of SPG3A mutant or dominant-negative atlastin proteins lacking GTPase activity causes prominent inhibition of ER reticularization, suggesting a role for atlastin GTPases in the formation of three-way junctions in the ER. However, secretory pathway trafficking as assessed using vesicular stomatitis virus G protein fused to green fluorescent protein (VSVG-GFP) as a reporter was essentially normal in both knockdown and dominant-negative overexpression conditions for all atlastins. Thus, the atlastin family of GTPases functions prominently in both ER and Golgi morphogenesis, but they do not appear to be required generally for anterograde ER-to-Golgi trafficking. Abnormal morphogenesis of the ER and Golgi resulting from mutations in atlastin-1 may ultimately underlie SPG3A by interfering with proper membrane distribution or polarity of the long corticospinal motor neurons.
Figures








References
-
- Fink J.K. Hereditary spastic paraplegia. Curr. Neurol. Neurosci. Rep. 2006;6:65–76. - PubMed
-
- Soderblom C., Blackstone C. Traffic accidents: molecular genetic insights into the pathogenesis of the hereditary spastic paraplegias. Pharmacol. Ther. 2006;109:42–56. - PubMed
-
- Züchner S. The genetics of hereditary spastic paraplegia and implications for drug therapy. Expert Opin. Pharmacother. 2007;8:1433–1439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

