The tumour-associated antigen L6 (L6-Ag) is recruited to the tetraspanin-enriched microdomains: implication for tumour cell motility
- PMID: 18270265
- PMCID: PMC2396850
- DOI: 10.1242/jcs.020347
The tumour-associated antigen L6 (L6-Ag) is recruited to the tetraspanin-enriched microdomains: implication for tumour cell motility
Abstract
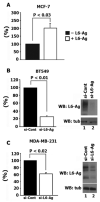
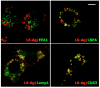
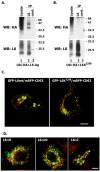
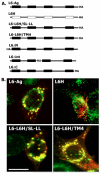
Tumour-associated antigen L6 (L6-Ag, also known as TM4SF1) regulates tumour cell motility and invasiveness. We found that L6-Ag is abundant on the plasma membrane and on intracellular vesicles, on which it is co-localised with the markers for late endosomal/lysosomal compartments, including Lamp1/Lamp2 proteins and LBPA. Antibody internalisation and live-imaging experiments suggested that L6-Ag is targeted to late endocytic organelles (LEO) predominantly via a biosynthetic pathway. Mapping experiments showed that the presence of transmembrane regions is sufficient for directing L6-Ag to LEO. On the plasma membrane, L6-Ag is associated with tetraspanin-enriched microdomains (TERM). All three predicted cytoplasmic regions of L6-Ag are crucial for the effective recruitment of the protein to TERM. Recruitment to TERM correlated with the pro-migratory activity of L6-Ag. Depletion of L6-Ag with siRNA has a selective effect on the surface expression of tetraspanins CD63 and CD82. By contrast, the expression levels of other tetraspanins and beta1 integrins was not affected. We found that L6-Ag is ubiquitylated and that ubiquitylation is essential for its function in cell migration. These data suggest that L6-Ag influences cell motility via TERM by regulating the surface presentation and endocytosis of some of their components.
Figures
Similar articles
-
CD63 tetraspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells.Blood. 2004 Aug 15;104(4):1183-90. doi: 10.1182/blood-2004-01-0104. Epub 2004 May 6. Blood. 2004. PMID: 15130945
-
Trafficking and function of the tetraspanin CD63.Exp Cell Res. 2009 May 15;315(9):1584-92. doi: 10.1016/j.yexcr.2008.09.020. Epub 2008 Oct 7. Exp Cell Res. 2009. PMID: 18930046 Review.
-
Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins.J Virol. 2008 Jan;82(2):1021-33. doi: 10.1128/JVI.01044-07. Epub 2007 Nov 7. J Virol. 2008. PMID: 17989173 Free PMC article.
-
CD82 endocytosis and cholesterol-dependent reorganization of tetraspanin webs and lipid rafts.FASEB J. 2009 Oct;23(10):3273-88. doi: 10.1096/fj.08-123414. Epub 2009 Jun 4. FASEB J. 2009. PMID: 19497983 Free PMC article.
-
Tetraspanins: push and pull in suppressing and promoting metastasis.Nat Rev Cancer. 2009 Jan;9(1):40-55. doi: 10.1038/nrc2543. Epub 2008 Dec 11. Nat Rev Cancer. 2009. PMID: 19078974 Review.
Cited by
-
Differential regulation of cellular functions by the C-termini of transmembrane 4 L six family proteins in 2- or 3-dimensional environment.Oncotarget. 2017 Feb 21;8(8):13277-13292. doi: 10.18632/oncotarget.14809. Oncotarget. 2017. PMID: 28129652 Free PMC article.
-
TM4SF1: a tetraspanin-like protein necessary for nanopodia formation and endothelial cell migration.Angiogenesis. 2011 Sep;14(3):345-54. doi: 10.1007/s10456-011-9218-0. Epub 2011 May 29. Angiogenesis. 2011. PMID: 21626280 Free PMC article.
-
Identification of three hub genes related to the prognosis of idiopathic pulmonary fibrosis using bioinformatics analysis.Int J Med Sci. 2022 Aug 15;19(9):1417-1429. doi: 10.7150/ijms.73305. eCollection 2022. Int J Med Sci. 2022. PMID: 36035368 Free PMC article.
-
Expression and Subcellular Distribution of GFP-Tagged Human Tetraspanin Proteins in Saccharomyces cerevisiae.PLoS One. 2015 Jul 28;10(7):e0134041. doi: 10.1371/journal.pone.0134041. eCollection 2015. PLoS One. 2015. PMID: 26218426 Free PMC article.
-
Single-cell Long Non-coding RNA Landscape of T Cells in Human Cancer Immunity.Genomics Proteomics Bioinformatics. 2021 Jun;19(3):377-393. doi: 10.1016/j.gpb.2021.02.006. Epub 2021 Jul 18. Genomics Proteomics Bioinformatics. 2021. PMID: 34284134 Free PMC article.
References
-
- Berditchevski F. Complexes of tetraspanins with integrins:more than meets the eye. J. Cell Sci. 2001;115:4143–4151. - PubMed
-
- Berditchevski F, Bazzoni G, Hemler ME. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J. Biol. Chem. 1995;270:17784–17790. - PubMed
-
- Berditchevski F, Chang S, Bodorova J, Hemler ME. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J. Biol. Chem. 1997;272:29174–29180. - PubMed
-
- Berditchevski F, Odintsova E, Sawada S, Gilbert E. Expression of the palmitoylation-deficient CD151 weakens the association of alpha 3 beta 1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signaling. J. Biol. Chem. 2002;277:36991–37000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

