Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion
- PMID: 18270681
- PMCID: PMC2270364
- DOI: 10.1007/s00125-007-0916-5
Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion
Abstract
Aims/hypothesis: Insulin secretion in pancreatic islets is dependent upon mitochondrial function and production of ATP. The transcriptional coactivator peroxisome proliferator activated receptor gamma coactivator-1 alpha (protein PGC-1alpha; gene PPARGC1A) is a master regulator of mitochondrial genes and its expression is decreased and related to impaired oxidative phosphorylation in muscle from patients with type 2 diabetes. Whether it plays a similar role in human pancreatic islets is not known. We therefore investigated if PPARGC1A expression is altered in islets from patients with type 2 diabetes and whether this expression is influenced by genetic (PPARGC1A Gly482Ser polymorphism) and epigenetic (DNA methylation) factors. We also tested if experimental downregulation of PPARGC1A expression in human islets influenced insulin secretion.
Methods: The PPARGC1A Gly482Ser polymorphism was genotyped in human pancreatic islets from 48 non-diabetic and 12 type 2 diabetic multi-organ donors and related to PPARGC1A mRNA expression. DNA methylation of the PPARGC1A promoter was analysed in pancreatic islets from ten type 2 diabetic and nine control donors. Isolated human islets were transfected with PPARGC1A silencing RNA (siRNA).
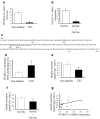
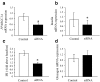
Results: PPARGC1A mRNA expression was reduced by 90% (p<0.005) and correlated with the reduction in insulin secretion in islets from patients with type 2 diabetes. After downregulation of PPARGC1A expression in human islets by siRNA, insulin secretion was reduced by 41% (p <or= 0. 01). We were able to ascribe reduced PPARGC1A expression in islets to both genetic and epigenetic factors, i.e. a common PPARGC1A Gly482Ser polymorphism was associated with reduced PPARGC1A mRNA expression (p<0.00005) and reduced insulin secretion (p<0.05). In support of an epigenetic influence, the PPARGC1A gene promoter showed a twofold increase in DNA methylation in diabetic islets compared with non-diabetic islets (p<0.04).
Conclusions/interpretation: We have shown for the first time that PPARGC1A might be important in human islet insulin secretion and that expression of PPARGC1A in human islets can be regulated by both genetic and epigenetic factors.
Figures
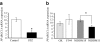
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.cmet.2005.05.004', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.cmet.2005.05.004'}, {'type': 'PubMed', 'value': '16054085', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16054085/'}]}
- Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/ng1180', 'is_inner': False, 'url': 'https://doi.org/10.1038/ng1180'}, {'type': 'PubMed', 'value': '12808457', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12808457/'}]}
- Mootha VK, Lindgren CM, Eriksson KF et al (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s001250100032', 'is_inner': False, 'url': 'https://doi.org/10.1007/s001250100032'}, {'type': 'PubMed', 'value': '11793024', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11793024/'}]}
- Ek J, Andersen G, Urhammer SA et al (2001) Mutation analysis of peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1) and relationships of identified amino acid polymorphisms to type II diabetes mellitus. Diabetologia 44:2220–2226 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s00125-005-0130-2', 'is_inner': False, 'url': 'https://doi.org/10.1007/s00125-005-0130-2'}, {'type': 'PubMed', 'value': '16435105', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16435105/'}]}
- Barroso I, Luan J, Sandhu MS et al (2006) Meta-analysis of the Gly482Ser variant in PPARGC1A in type 2 diabetes and related phenotypes. Diabetologia 49:501–505 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC525741', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC525741/'}, {'type': 'PubMed', 'value': '15546003', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15546003/'}]}
- Ling C, Poulsen P, Carlsson E et al (2004) Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest 114:1518–1526 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

