Connections of cat auditory cortex: I. Thalamocortical system
- PMID: 18271026
- PMCID: PMC2658654
- DOI: 10.1002/cne.21611
Connections of cat auditory cortex: I. Thalamocortical system
Abstract
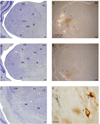
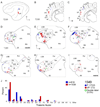
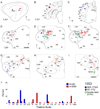
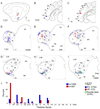
Despite the functional importance of the medial geniculate body (MGB) in normal hearing, many aspects of its projections to auditory cortex are unknown. We analyzed the MGB projections to 13 auditory areas in the cat using two retrograde tracers to investigate thalamocortical nuclear origins, topography, convergence, and divergence. MGB divisions and auditory cortex areas were defined independently of the connectional results using architectonic, histochemical, and immunocytochemical criteria. Each auditory cortex area received a unique pattern of input from several MGB nuclei, and these patterns of input identify four groups of cortical areas distinguished by their putative functional affiliations: tonotopic, nontonotopic, multisensory, and limbic. Each family of areas received projections from a functionally related set of MGB nuclei; some nuclei project to only a few areas (e.g., the MGB ventral division to tonotopic areas), and others project to all areas (e.g., the medial division input to every auditory cortical area and to other regions). Projections to tonotopic areas had fewer nuclear origins than those to multisensory or limbic-affiliated fields. All projections were organized topographically, even those from nontonotopic nuclei. The few divergent neurons (mean: 2%) are consistent with a model of multiple segregated streams ascending to auditory cortex. The expanded cortical representation of MGB auditory, multisensory, and limbic affiliated streams appears to be a primary facet of forebrain auditory function. The emergence of several auditory cortex representations of characteristic frequency may be a functional multiplication of the more limited maps in the MGB. This expansion suggests emergent cortical roles consistent with the divergence of thalamocortical connections.
(c) 2008 Wiley-Liss, Inc.
Figures







References
-
- Aitkin LM, Dunlop CW. Interplay of excitation and inhibition in the cat medial geniculate body. J Neurophysiol. 1968;31:44–61. - PubMed
-
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: evidence for two largely segregated systems of connections. J Comp Neurol. 1980;194:663–701. - PubMed
-
- Basbaum AI, Menetrey D. Wheat germ agglutinin-apoHRP gold: a new retrograde tracer for light- and electron-microscopic single- and double-label studies. J Comp Neurol. 1987;261:306–318. - PubMed
-
- Benedek G, Fischer-Szátmári L, Kovács G, Perényi J, Katoh YY. Visual, somatosensory and auditory modality properties along the feline suprageniculate-anterior ectosylvian sulcus/insular pathway. Prog Brain Res. 1996;90:325–334. - PubMed
-
- Berman AL, Jones EG. The Thalamus and Basal telencephalon of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison: University of Wisconsin Press; 1982.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

